“In the 200 years that we have known about NMO, this year has been the most exciting by far”, said Dr Stacey Clardy (University of Utah, Salt Lake City, USA) in a review session on NMOSD. Her enthusiasm was largely based on 3 monoclonal antibodies in late-stage development for the treatment of NMOSD. Dr Clardy summarised the results of all 3 therapies, that had been presented earlier during the meeting.
Eculizumab
In a randomised, double-blind, time-to-event trial (PREVENT), eculizumab had a significantly lower risk of relapse than placebo among 143 patients with AQP4-IgG–positive NMOSD [1]. The safety profile was similar to that in other indications.
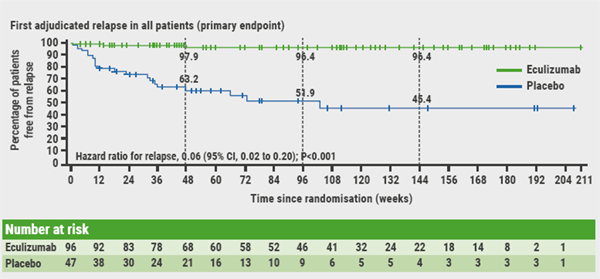
Eculizumab exerts its effect by inhibiting C5 complement activation and subsequent assembly of the membrane attack complex, which is instrumental in effecting astrocytic injury in NMOSD patients. Dr Clardy pointed out that patients were severely ill; they were allowed supportive immunosuppressive therapy. A total of 143 patients were randomised 2:1 to eculizumab (1,200 mg every 2 weeks maintenance) or placebo. As planned, the study was stopped at 23 adjudicated on-trial relapses. Of these, 3 occurred in the eculizumab group and 20 in the placebo group, representing a 94.2% reduction in risk of relapse compared with placebo (HR 0.06; P<0.0001; see Figure). After 48 weeks, 97.9% and 63.2% of patients were relapse-free, respectively. The annualised relapse rate was significantly lower with eculizumab than placebo (0.016 vs 0.350; P<0.0001). The treatment effect was observed regardless of whether or not the patients had received concomitant immunosuppressive agents. Most treatment-related adverse events were mild to moderate.
Figure: Analyses of time to first adjudicated relapse [1]
Inebilizumab
In a phase 3, double-masked, placebo-controlled trial (N-MOmentum), inebilizumab was associated with a 77% reduction in risk of relapse in AQP4-IgG seropositive patients (HR 0.227; P<0.0001). The effect on the total population, including AQP4-IgG seronegative patients was similar with a reduction of 73% (HR 0.272; P<0.0001) [2].
Inebilizumab is an anti-CD19 depleting B cell monoclonal antibody. In the inebilizumab study, 231 NMOSD patients (over 90% seropositive for anti-AQP4 antibodies) study were randomised 3:1 to inebilizumab or placebo for 6.5 months. Concurrent treatment with other immunosuppressants was prohibited. After 28 weeks, 89% of actively treated AQP4-IgG seropositive patients were attack-free, versus 58% in the placebo group. On this basis, Prof. Bruce Cree (University of California, San Francisco, USA) explained that further recruitment into the placebo arm was deemed unethical as 43 relapses were observed among the 231 randomised patients. Inebilizumab also demonstrated statistically significant benefits in key secondary endpoints, including reduction in worsening from baseline in Expanded Disability Status Scale (EDSS) scores (P=0.0049); NMOSD-related hospitalisations (P=0.01); and frequency of cumulative total active MRI lesions (P=0.0034). Inebilizumab had a favourable safety and tolerability profile, with an adverse event rate similar to placebo.
Satralizumab
A subgroup analysis of the SAkuraSky study showed that satralizumab is especially effective in reducing relapses in NMO/NMOSD AQP4-antibody-positive patients [3].
Satralizumab is a recycling anti-IL-6 receptor monoclonal antibody. At baseline, 66.3% of patients were AQP4-antibody-positive. In this subgroup, satralizumab showed a 79% risk reduction of relapses compared to placebo (HR 0.21). After 48 and 96 weeks, 91.5% and 91.5% were relapse free with satralizumab, and 59.9% and 53.3% with placebo, respectively. In NMO/NMOSD AQP4-antibody-negative patients, satralizumab reduced the risk of relapse by 34% compared to placebo (HR 0.66). After 48 and 96 weeks, 84.4% and 56.3% were relapse free with satralizumab, and 75.5% and 67.1% with placebo. Satralizumab had a favourable benefit-risk profile.
1. Pittock SJ, et al. N Engl J Med. 2019 May 3.
2. Cree B, et al. AAN 2019, Plen02.001.
3. Yamamura T, et al. AAN 2019, S43.008.
Posted on
Previous Article
« New AAN guideline for treating Tourette syndrome Next Article
Amyloid PET in cognitively impaired patients »
« New AAN guideline for treating Tourette syndrome Next Article
Amyloid PET in cognitively impaired patients »
Table of Contents: AAN 2019
Featured articles
Letter from the Editor
Interview with Prof. Natalia Rost
Alzheimer's Disease and other Dementias
Amyloid PET in cognitively impaired patients
Tight blood pressure control lowers risk of mild cognitive impairment
Epilepsy
Headache and Migraine
Multiple Sclerosis and NMOSD
Immune tolerance by peptide-loaded tolerogenic dendritic cells
Biotin, ocrelizumab, and ibudilast in progressive MS
No increased MS relapse risk postpartum
Neuromuscular Disorders
First-ever effective and safe treatment of CMT1A
Parkinson’s Disease and other Movement Disorders
Leukaemia and hypertension therapies tested in Parkinson’s disease
Stroke
Miscellaneous
Possibly lifesaving therapy in refractory PML
New AAN guideline for treating Tourette syndrome
Subspecialty teleneurology: feasible and highly valued
Related Articles

July 30, 2019
Interview with Prof. Natalia Rost
July 30, 2019
Levodopa oral inhalation powder
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

