Some COVID-19 patients may develop fibrotic abnormalities. Patients with severe acute disease are particularly at risk [1].
At present, the long-term pulmonary consequences of COVID-19 remain speculative [2]. “However, we know that the severity of COVID-19 is related to symptoms at discharge,” said Prof. Francesco Blasi (University of Milan, Italy). Particularly patients who suffer from severe COVID-19 disease which requires admission in an intensive care unit seem to be at risk. In the first large series of hospitalised patients with COVID-19 in Wuhan (China), chest computer tomography (CT) showed bilateral ground glass opacities with or without consolidation and with lower lobe predilection in all patients [2,3]. Although the virus is eradicated in patients who have recovered from COVID-19, this does not, in itself, preclude the development of progressive, fibrotic irreversible interstitial lung disease.
Prof. Blasi discussed an early analysis of discharged COVID-19 patients (enrolled between 5 February and 17 March 2020); 47% of patients showed impaired diffusing capacity of the lungs for carbon monoxide (DLCO) and 25% had a reduced total lung capacity (TLC). Impaired diffusion capacity was found in 30.4% of patients with mild disease, 42.4% of patients with pneumonia, and 84.2% of patients that suffered from severe pneumonia. “Not only impaired diffusion, but also CT changes are noticed. In particular in patients with severe disease,” Prof. Blasi said [1,4].
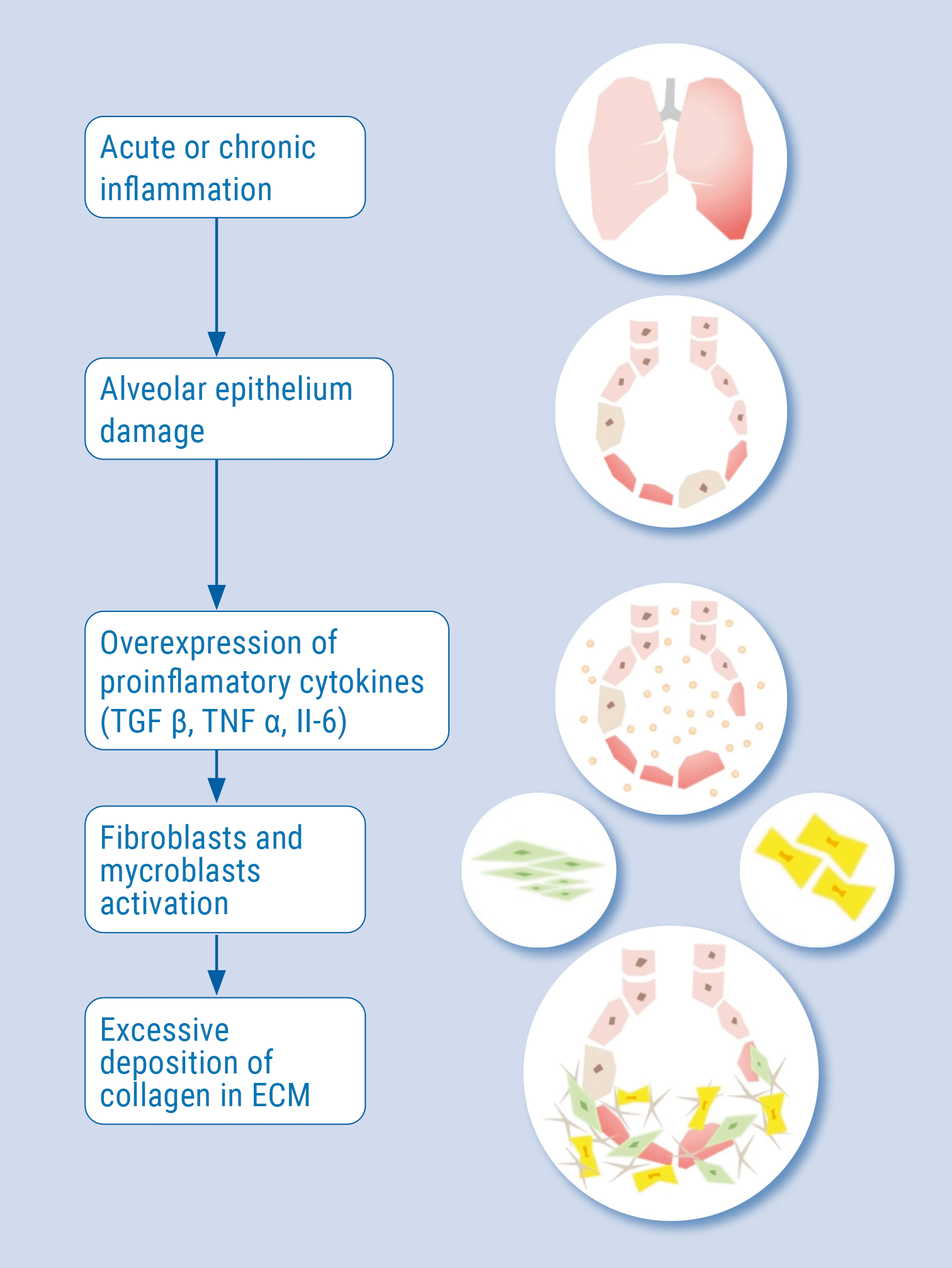
The correlation between severe pneumonia during a COVID-19 infection and secondary fibrosis can be explained by acute or chronic inflammation that leads to damage of the alveolar epithelium. In this state, proinflammatory cytokines such as TGFβ, TNFa, and IL-6 are released. They activate fibroblasts and myofibroblasts, which leads to excessive deposition of collagen and other extracellular matrix components, as well as the destruction of normal lung architecture. The similar cytokine profiles in idiopathic pulmonary fibrosis and COVID-19 suggest an analogous pathomechanism of pulmonary fibrosis in those diseases (see Figure) [5]. Viral infections may act as triggers for the initiation of lung fibrosis or promote exacerbations of existing lung fibrosis. Especially elderly male patients are prone to viral-induced fibrosis due to immunosenescence.
Figure: The pathogenesis of pulmonary fibrosis. Modified from [5]
With regard to therapy of SARS-CoV-2-associated pulmonary fibrosis, anything from traditional Chinese medicine to steroids and antifibrotics has been considered. “Steroids seem to work according to our experience: they improve and reduce pulmonary involvement and also improve exercise tolerance. But I think antifibrotics could be another interesting approach,” concluded Prof. Blasi.
- Blasi S. Long-term impact the lung and beyond. COV3651, ERS International Virtual Congress 2020, 7-9 Sept.
- Spagnolo P et al. Lancet Respir Med 2020;8:750-72.
- Wang D, et al. JAMA 2020;323:1061-1069.
- Hu Q, et al. Eur J Radiol 2020; 128:109017.
- Lechowicz K, et a.l J. Clin. Med. 2020;9:1917.
Posted on
Previous Article
« IL-5 antagonist showed efficacy in chronic rhinosinusitis with nasal polyps Next Article
First-in-class P2X3 receptor antagonist shows promise for chronic cough treatment »
« IL-5 antagonist showed efficacy in chronic rhinosinusitis with nasal polyps Next Article
First-in-class P2X3 receptor antagonist shows promise for chronic cough treatment »
Table of Contents: ERS 2020
Featured articles
COVID-19 and the Lung
COVID-19 infections: Bronchoscopy provides additional diagnostic certainty
COVID-19 vaccines: An ongoing race
COVID-19: What is the risk of reinfection?
COVID-19 App: The Dutch experience
Secondary pulmonary fibrosis: a possible long-term effect of severe COVID-19
COVID-19 survivors benefit from structured follow-up
Early pulmonary rehabilitation post-COVID-19 aids recovery
Asthma – What's New
Mild asthma: A fundamental change in management
Dupilumab shows long-term efficacy in asthma patients
Severe asthma: Oral corticosteroids maintenance therapy associated with toxicity
First-in-class tyrosine kinase inhibitor shows promise in severe asthma
Predicting individual effectiveness of biologics in severe asthma
IL-5 antagonist showed efficacy in chronic rhinosinusitis with nasal polyps
Treatment according to genotype: The future of asthma therapy?
COPD – The Beat Goes On
The role of chronic symptoms as early biomarkers of COPD development
Urgent call for studies in COPD patients aged 40-60 years
Nasal high-flow therapy: a novel treatment option for hypercapnic COPD patients
Exacerbation history is a reliable predictor of future exacerbations
Singing training effective as physical rehabilitation in COPD
Current prediction tools underestimate exacerbation risk of severe COPD patients
Exercise and Sleep: From Impaired Function to New Therapeutic Strategies
CPAP withdrawal has negative consequences for sleep apnoea patients
Physical activity improves AHI in sleep apnoea patients
The Tobacco Epidemic: From Vaping to Cannabis
Poly-use of nicotine products and cannabis: a deadly combination
E-cigarettes: A source of chronic lung inflammation
Social smoking: Do not underestimate the risks
Chronic Cough – State of the Art
LEAD study shows multiple phenotypes in many chronic cough patients
First-in-class P2X3 receptor antagonist shows promise for chronic cough treatment
Lung Cancer Detection
Lung cancer screening: Most patients not eligible 1-2 years prior to diagnosis
Distinct changes in lung microbiome precede clinical diagnosis of lung cancer
Best of Posters
Smartphone-based cough detection helpful in predicting asthma deterioration
Reduced lung function associated with cognitive decline in the elderly
Longer hospital stay and fewer transplants for frail ILD patients
Related Articles

June 22, 2022
EULAR 2022 Highlights Podcast
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

