https://doi.org/10.55788/41f02088
Findings from the EU-funded, randomised, open-label SECURE trial (NCT02596126) were presented by Dr Valentin Fuster (Mount Sinai Health System, NY, USA), and simultaneously published in the New England Journal of Medicine [1,2]. It is the first trial testing the efficacy of a fixed-dose combination polypill for secondary cardiovascular prevention in individuals ≥65 years old.
SECURE aimed to evaluate the potential benefit of the polypill as a component of a cost-effective, globally available, and comprehensive treatment strategy for secondary prevention of cardiovascular events (death from cardiovascular causes, non-fatal MI, stroke, and hospitalisation requiring revascularisation) as compared with standard therapy (the 3 components of the polypill given separately). "Although most patients initially adhere to treatment after an acute event such as an infarction, adherence drops off after the first few months. Our goal was to have an impact right from the start, and most of the patients in the study began taking a simple polypill in the first week after having a heart attack," Dr Fuster explained.
The trial randomised 2,499 patients to receive either the polypill or the usual care of 3 separate tablets. The polypill intervention contained aspirin (100 mg), ramipril (2.5, 5, or 10 mg), and atorvastatin (20 or 40 mg). The baseline characteristics were similar in each arm of the study.
The primary endpoint was a composite of 4 major cardiovascular events: death from cardiovascular causes, non-fatal MI, non-fatal stroke, and need for emergency coronary revascularisation. After a median of 36 months of follow-up, 9.5% of participants in the polypill arm and 12.7% of the usual care arm had experienced a primary outcome event, which translated to a 24% lower risk of a primary-outcome event in favour of the polypill over the 3 separate drugs (HR 0.76; 95% CI 0.60–0.96; P=0.02, see Figure). Likewise, a key secondary outcome event (composite of cardiovascular death, non-fatal type 1 MI, or non-fatal ischaemic stroke) occurred in 8.2% of the participants in the polypill arm compared with 11.7% in the usual-care arm (HR 0.70; 95% CI 0.54–0.90; P=0.005). Strikingly, for the single component cardiovascular-related death, a relative reduction of 33% in the polypill group supported a clear clinical benefit. Findings showed that participants in the polypill group had a higher level of treatment adherence than those in the control group. The improved adherence supported the earlier findings of the FOCUS study [3]; good adherence may explain the improved outcomes of those taking the polypill. The results were consistent across prespecified subgroups. Adverse events were similar between groups.
Figure: Primary endpoint analysis of SECURE, composite of CV death, MI, stroke, and urgent revascularisation [1]
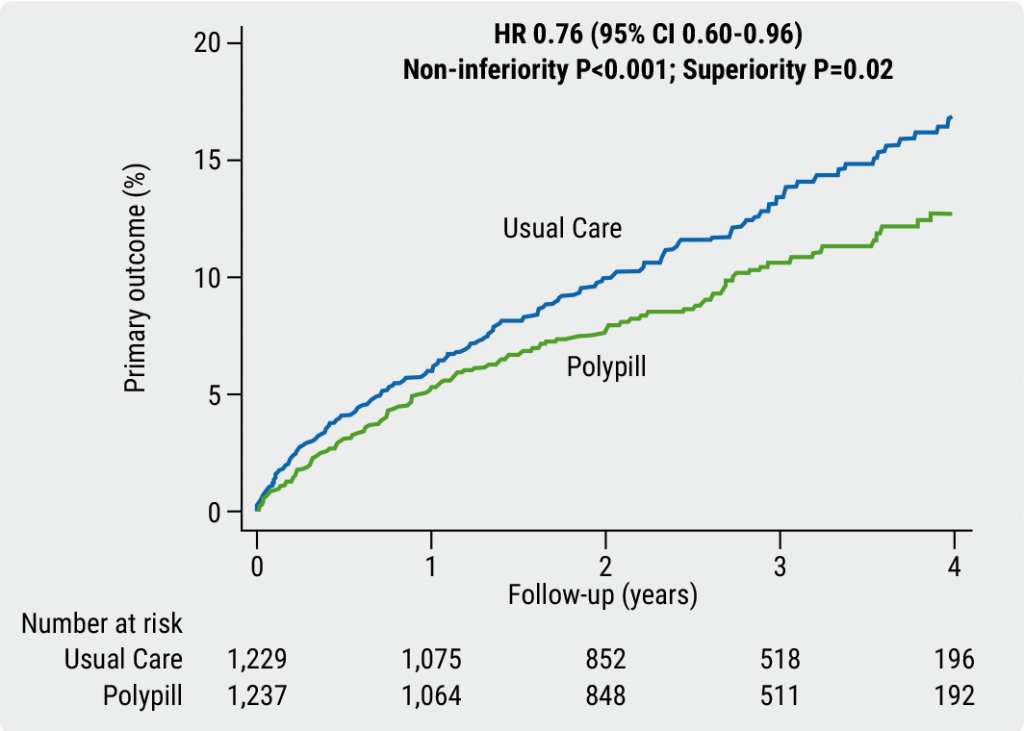
"The SECURE study findings suggest that the polypill could become an integral element of strategies to prevent recurrent cardiovascular events in patients who have had a heart attack. By simplifying treatment and improving adherence, this approach has the potential to reduce the risk of recurrent cardiovascular disease and death on a global scale," concluded Dr Fuster.
- Fuster V, et al. A polypill strategy in secondary prevention: results of the SECURE trial. Hot Line Session 1, ESC Congress 2022, Barcelona, Spain, 26–29 August.
- Castellano JM et al. N Engl J Med. 2022. Online 26 Aug 2022. 10.1056/NEJMoa2208275.
- Castellano JM, et al. J Am Coll Cardiol. 2014;64(20):2071–82.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Long-term therapy with evolocumab associated with lower CV mortality Next Article
Danish study suggests starting CVD screening before age 70 »
« Long-term therapy with evolocumab associated with lower CV mortality Next Article
Danish study suggests starting CVD screening before age 70 »
Table of Contents: ESC 2022
Featured articles
ESC Clinical Practice Guidelines
Prevention of VT and sudden cardiac death: the new recommendations
New and first ESC cardio-oncology guideline
The 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension
Cardiovascular assessment and management of patients undergoing non-cardiac surgery
Heart Failure
Old dogs, new tricks: Acetazolamide plus loop diuretics improves decongestion
No effect of neprilysin inhibition on cognition
Dapagliflozin DELIVERs for HFmrEF/HFpEF
Meta-analysis of DELIVER and EMPEROR-Preserved
Anticoagulation
Rheumatic heart disease-associated AF: standard-of-care holds ground
New anticoagulant safe and maybe effective: PACIFIC-AMI and PACIFIC-Stroke outcomes
AXIOMATIC-SSP: Reducing risk of ischaemic stroke with factor XIa inhibition?
Evolving evidence for P2Y12 inhibition in chronic coronary syndromes: PANTHER
Prevention
Danish study suggests starting CVD screening before age 70
Polypill SECUREs win in secondary prevention in elderly
Long-term therapy with evolocumab associated with lower CV mortality
ARBs + beta-blockers may delay Marfan syndrome aortic root replacement
ENTRIGUE: Subcutaneous pegozafermin in severe hypertriglyceridaemia
Artificial Intelligence & Digital Health – What Is New
First RCT evidence for use of AI in daily practice
AI-enhanced echography supports aortic stenosis patients
Ischaemia
Medical therapy versus PCI for ischaemic cardiomyopathy
Allopurinol disappoints in ALL-HEART
Conservative or invasive management for high-risk kidney disease patients with ischaemia?
Genotype-guided antiplatelet therapy in patients receiving PCI
Other HOTLINE Sessions
BOXing out oxygen and blood pressure targets
Coronary CT angiography diagnostics compared head-to-head
High-dose influenza vaccine: mortality benefit?
FFR-guided decision-making in patients with AMI and multivessel disease
Related Articles
September 8, 2020
TWILIGHT sub-study: same outcomes for diabetes patients
October 27, 2022
Danish study suggests starting CVD screening before age 70
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

