https://doi.org/10.55788/b6122478
Dr Niklas Dyrby Johansen (Herlev and Gentofte Hospital, Denmark) pointed out that several previous studies have indicated that influenza infection is strongly associated with heart failure and myocardial infarction (MI) hospitalisations, coupled with evidence that influenza vaccination can prevent cardiovascular events [1–3]. The DANFLU-1 study (NCT05048589) aimed to address the unmet need that to date, no randomised trial has assessed the effects of high-dose, compared with standard-dose, quadrivalent influenza vaccines on severe clinical outcomes such as hospitalisations and mortality in a general population of older adults which would conclusively determine the potential public health value of high-dose vaccines [4]. However, to achieve this goal, a very large sample size would be required.
DANFLU-1 was therefore conducted as a feasibility trial, which integrated a pragmatic open-label randomised trial into the official Danish vaccination programme. The primary endpoint was feasibility (e.g. number of participants included and randomised to each vaccine, agreement between randomisation assignment and actual received vaccine, the balance between groups in terms of number of subjects in each arm and baseline characteristics, and a comparison of baseline characteristics with the overall Danish general population aged 65 to 79 years). The secondary endpoint was to determine the relative vaccine effectiveness (rVE) of the high-dose compared with the standard-dose for cardiovascular and respiratory endpoints.
Participants aged 65 to 79 years were randomised 1:1 to either high-dose (n=6,245) or standard-dose (n=6,232) influenza vaccine. Baseline characteristics were comparable with the overall Danish population aged 65 to 79 years.
The primary endpoint for feasibility was met; registry-based data collection and complete follow-up information was available for >99.9% of participants. The initial efficacy results were promising: High-dose recipients had a lower incidence of hospitalisation for influenza or pneumonia (0.2%) compared with standard-dose recipients (0.4%) and a rVE of 64.4% (95% CI 24.4–84.6; P<0.001, see Figure). High-dose recipients also had a lower incidence of all-cause mortality (0.3% vs 0.7%, respectively) and a rVE of 48.9% (95% CI 11.5–71.3; P<0.001). There were no significant differences in serious adverse events between the different doses.
Figure: Clinical outcomes of DANFLU-1 [4]
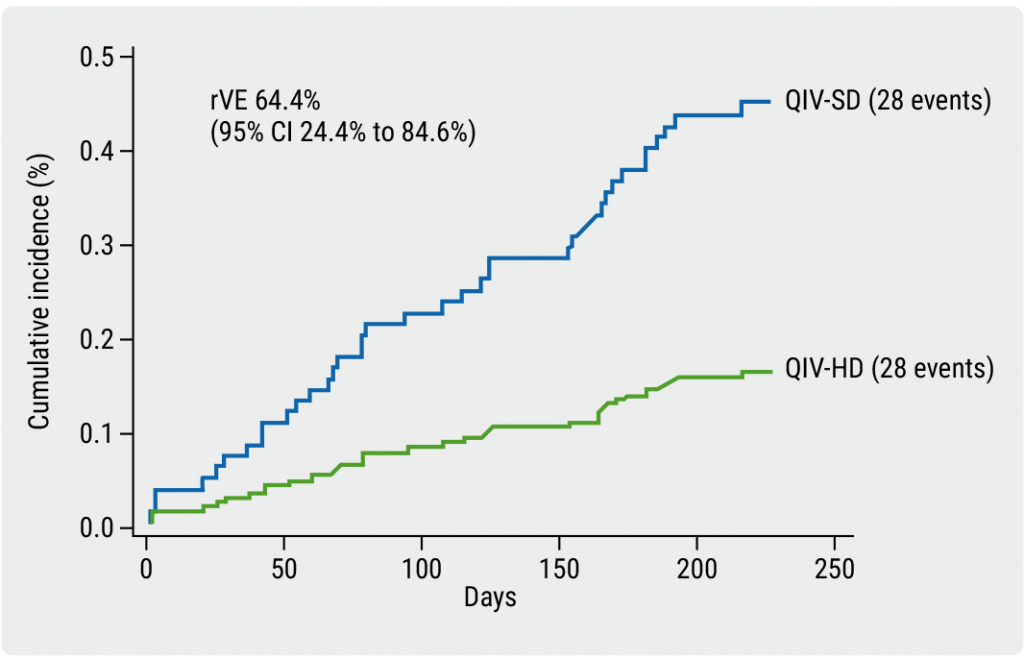
QIV, quadrivalent influenza vaccine; rVE, relative vaccine effectiveness; SD, standard dose; HD, high dose
The researchers concluded that integrating an influenza vaccine trial into the Danish vaccination programme was feasible, and estimate that a fully powered trial to establish superiority of high-dose influenza vaccines will require a sample size of approximately 200,000 participants.
- Kwong JC, et al. N Engl J Med. 2018;378(4):345–353.
- Kytömaa S, et al. JAMA Cardiol. 2019;4(4):363–369.
- Fröbert O, et al. Circulation. 2021;144(18):1476–1484.
- Johansen ND, et al. DANFLU-1 - High-dose vs. standard-dose quadrivalent influenza vaccine in elderly adults. Hot Line Session 2, ESC Congress 2022, Barcelona, Spain, 26–29 August.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« FFR-guided decision-making in patients with AMI and multivessel disease Next Article
Coronary CT angiography diagnostics compared head-to-head »
« FFR-guided decision-making in patients with AMI and multivessel disease Next Article
Coronary CT angiography diagnostics compared head-to-head »
Table of Contents: ESC 2022
Featured articles
ESC Clinical Practice Guidelines
Prevention of VT and sudden cardiac death: the new recommendations
New and first ESC cardio-oncology guideline
The 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension
Cardiovascular assessment and management of patients undergoing non-cardiac surgery
Heart Failure
Old dogs, new tricks: Acetazolamide plus loop diuretics improves decongestion
No effect of neprilysin inhibition on cognition
Dapagliflozin DELIVERs for HFmrEF/HFpEF
Meta-analysis of DELIVER and EMPEROR-Preserved
Anticoagulation
Rheumatic heart disease-associated AF: standard-of-care holds ground
New anticoagulant safe and maybe effective: PACIFIC-AMI and PACIFIC-Stroke outcomes
AXIOMATIC-SSP: Reducing risk of ischaemic stroke with factor XIa inhibition?
Evolving evidence for P2Y12 inhibition in chronic coronary syndromes: PANTHER
Prevention
Danish study suggests starting CVD screening before age 70
Polypill SECUREs win in secondary prevention in elderly
Long-term therapy with evolocumab associated with lower CV mortality
ARBs + beta-blockers may delay Marfan syndrome aortic root replacement
ENTRIGUE: Subcutaneous pegozafermin in severe hypertriglyceridaemia
Artificial Intelligence & Digital Health – What Is New
First RCT evidence for use of AI in daily practice
AI-enhanced echography supports aortic stenosis patients
Ischaemia
Medical therapy versus PCI for ischaemic cardiomyopathy
Allopurinol disappoints in ALL-HEART
Conservative or invasive management for high-risk kidney disease patients with ischaemia?
Genotype-guided antiplatelet therapy in patients receiving PCI
Other HOTLINE Sessions
BOXing out oxygen and blood pressure targets
Coronary CT angiography diagnostics compared head-to-head
High-dose influenza vaccine: mortality benefit?
FFR-guided decision-making in patients with AMI and multivessel disease
Related Articles
October 27, 2022
First RCT evidence for use of AI in daily practice
October 27, 2022
Meta-analysis of DELIVER and EMPEROR-Preserved
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

