Patients with an MRD-negative state appear to have the same prognosis, regardless of whether they have standard or high-risk disease. However, even though MRD-negativity can be achieved in around 50% of patients with standard risk disease, this figure is significantly lower in those with high-risk features, e.g. concomitant t(4;14) or del(17p) [2]. Therefore, the aim should be to increase the rate of MRD-negativity in high-risk patients. Although adding daratumumab can increase rates of MRD-negativity and improve PFS, this is still within a subset of patients without adverse cytogenetics. Thus, CAR-T therapy may be beneficial as a consolidation therapy based on their long persistence and could potentially replace maintenance therapy.
Several immunotherapy targets have been identified for CAR-T cells in MM with ongoing clinical trials investigating anti-CD19, anti-CD138, and anti-BCMA CAR-T cells with others targeting SLAMF7 (CARAMBA study: phase 1-2a study in the relapsed setting), CD38, and CD44v6-CAR (EURE-CART study). In the bb2121 trial, heavily pre-treated patients were enrolled and received anti-BCMA CAR T cells. The toxicity profile was milder than in the lymphoma CAR-T products and at the highest dose of >150 x 106 there was a 95.5% ORR with 50% CR. A median PFS of 17.7 months was also seen in 16 patients who were MRD-negative; all 33 patients (100%) tested MRD-negative at one or more time points [3]. The length of the PFS reported in this heavily pre-treated population was a notable advance.
Another ongoing trial is the LEGEND-2 study, using LCAR-B38M with two BCMA-targeting domains leading to high avidity binding. These patients had a median of 3 lines of prior therapy. The toxicity profile was consistent with other BCMA-targeted CAR-T cell therapy, with cytokine release syndrome (CRS) ≥grade 3 experienced by 7% of patients and neurotoxicity in 2%. With an average 12-month follow-up, in 57 patients, the median duration of response was 16 months (95% CI 12–not reached [NR]) and increased to 22 months (95% CI 14–NR) in MRD-negative patients [4].
Shi et al. presented updated data at ASH 2018 where they used both anti-BCMA and anti-CD19 CAR-T in patients with high-risk, heavily pre-treated MM. They showed MRD-negative status can be achieved in this dual approach, despite the high-risk features of the patients they were treating. This approach also showed a high efficacy with over 50% CR rate and a manageable safety profile [5].
Current CAR-T trials in MM have shown an improved safety profile when compared to the lymphoma setting with lower CRS and neurotoxicity rates. Additionally, even in a heavily pre-treated group of patients, there is a high rate of CR/MRD negativity. A study evaluating JCARH125 –a fully human binder with a low affinity for surface BCMA and a modified spacer to increase binding to BCMA– is ongoing, with the hope that decreasing the immunogenicity of CAR-T therapy will improve survival of the CAR-T cells, and therefore the ORR. Dr Chunrui Li (Huazhong University of Science and Technology, China) presented a study of patients receiving a fully humanised CAR-T CT103A [6]. As of the data cut-off date of 22 May 2019, the ORR was 100% (CR 64%, very good partial response 36%, median follow-up 40 weeks) with strong persistence and high expansion of the CAR-T in vivo (see Figure). All patients (100%) experienced cytokine release syndrome (CRS) within 2 to 5 days (median 2.6), which resolved within 14 days. CRS was routinely managed with tocilizumab and sometimes steroids. Interestingly, the 12-patient study included 4 patients having previously relapsed from a prior CAR-T therapy, a murine anti-BCMA CAR-T. “Relapsed/refractory multiple myeloma is associated with a poor prognosis,” said Dr Li. “Many who receive CAR-T treatments have relapsed, and with a non-human scFv, retreatment may not be an option due to immunogenicity. With a fully human BCMA scFv, CT103A provides an effective option for these patients. This data suggests they should not be excluded from future CAR-T trials.”
Figure: Efficacy data showing an ORR of 100%, CR of 64%, and a VGPR of 36% [6]
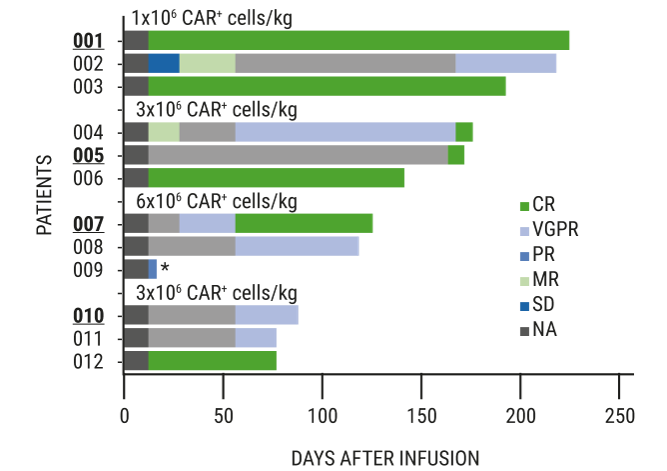 CR, complete response; MR, minimal response; NA, no assessment at time of data cut-off ; ORR, objective response rate; PR, partial response; SD, stable disease; VGPR, very good partial response.Bold and underlined patient numbers indicate patients having relapsed from a previous CAR-T therapy. Data cut-off date: 22 May 2019.
CR, complete response; MR, minimal response; NA, no assessment at time of data cut-off ; ORR, objective response rate; PR, partial response; SD, stable disease; VGPR, very good partial response.Bold and underlined patient numbers indicate patients having relapsed from a previous CAR-T therapy. Data cut-off date: 22 May 2019.Prof. Einsele pointed to the remaining challenges to tackle: lack of persistence and immunogenicity. Other causes for CAR-T cell therapy failing include malignant stem cells expressing different surface antigens in the bone marrow (which is rare) and the upregulation of inhibitory receptors. These resistance mechanisms are more pronounced in patients with a higher tumour load.
Based on the results presented at EHA, it could be argued that CAR-T therapy should be moved into an earlier line of therapy as using CAR-T upfront in high-risk patients has shown impressive efficacy results. If the toxicity profile can be shown to be acceptable, then CAR-T may be feasible for elderly patients who are ineligible for ASCT as an upfront consolidation therapy and potentially maintenance therapy. Beyond the second line, whilst CAR-T has been shown to be effective and provide a prolonged PFS, the cost-benefit ratio needs to be analysed. For patients who relapse early following ASCT and those with high-risk cytogenetics, CAR-T may provide a long-term PFS improvement.
- Einsele H. 24th Congress of the EHA, 13-16 June 2019, Amsterdam, the Netherlands.
- Arana P, et al. Leukemia. 2018 Apr;32(4):971-978.
- Raje N, et al. N Engl J Med. 2019 May 2;380(18):1726-1737.
- Zhao WH, et al. J Hematol Oncol. 2018 Dec 20; 11:141.
- Shi X, et al. Abstract 1009. ASH 60th Annual Meeting and Exposition, San Diego, CA.
- Li WH, et al. Abstract S827, 24th Congress of the EHA, 13-16 June 2019, Amsterdam, the Netherlands.
Posted on
Previous Article
« Gilteritinib prolongs overall survival in patients with FLT3-mutated relapsed/refractory AML Next Article
Unmutated IGHV as predictive factor for venetoclax/obinutuzumab benefit in frontline CLL »
« Gilteritinib prolongs overall survival in patients with FLT3-mutated relapsed/refractory AML Next Article
Unmutated IGHV as predictive factor for venetoclax/obinutuzumab benefit in frontline CLL »
Table of Contents: EHA 2019
Featured articles
Editor Biography
Interview with EHA President Prof. Pieter Sonneveld
Myeloid Malignancies
Residual disease in AML patients prior to stem cell transplant increases relapse risk
Gilteritinib prolongs overall survival in patients with FLT3-mutated relapsed/refractory AML
Initial data on AMV564 in patients with relapsed/refractory AML
Overcoming the “don’t eat me” signal in AML and MDS
Asciminib plus imatinib in patients with heavily pre-treated chronic myeloid leukaemia
Guadecitabine vs treatment of choice in AML
Lymphoid Malignancies
Unmutated IGHV as predictive factor for venetoclax/obinutuzumab benefit in frontline CLL
CAR-T cell therapy in ALL as breakthrough advance
Brentuximab vedotin continues to demonstrate superior clinical activity in classical Hodgkin lymphoma
Infectious complications mild and not common in patients receiving CAR-T therapy for diffuse large B cell lymphoma
Obinutuzumab/polatuzumab in follicular lymphoma
Exciting survival data for ibrutinib vs placebo in treatment-naïve, early-stage CLL
ASCEND study: Acalabrutinib improves progression-free survival in relapsed/refractory CLL
Venetoclax-obinutuzumab combination elicits high response rates in CLL
Myeloma
CASSIOPEIA trial: Phase 3 results of daratumumab + bortezomib/thalidomide/dexamethasone in multiple myeloma
Chimeric antigen receptor T cell therapy in multiple myeloma
Higher levels of treatment satisfaction without compromising efficacy: subcutaneous daratumumab in RRMM
Adding isatuximab to pomalidomide and dexamethasone improves PFS and ORR in RRMM
Subcutaneous daratumumab + cyclophosphamide, bortezomib, and dexamethasone in patients with newly diagnosed amyloid light chain amyloidosis
Venetoclax for multiple myeloma: effective but some safety concerns
Benign Haematology
New sickle cell drug voxelotor boosts levels of haemoglobin
Positive initial data evaluating the safety and efficacy of IMR-687 for treatment of sickle cell disease
Haematopoietic stem cell transplantation improves stroke risk in children with sickle cell anaemia
Early trial data shows positive results for treating anaemia in patients with end-stage renal failure
Bench-to-Bedside
Transformation of foetal haematopoietic stem and progenitor cells in the background of trisomy 21
Treating thalassemia twice, in mice
Haematopoietic stem cells can sense tissue damage in the gut
Promising news for gene therapy for sickle cell disease
Related Articles
August 9, 2019
Guadecitabine vs treatment of choice in AML
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

