Dr Jorge Maspero (Fundación CIDEA, Argentina) presented the research in a late-breaking clinical trial session, pointing out that asthma and chronic rhinosinusitis with nasal polyps (CRSwNP) are frequently comorbid conditions with up to 67% of patients with CRSwNP having asthma as well. CRSwNP is a chronic type 2 inflammatory disease with a high symptom burden and poor quality of life. It is characterised by an inflammatory signature involving interleukin(IL)-4, IL-5, and IL-13, with prominent tissue infiltration by eosinophils, lymphocytes, basophils, and mast cells.
Both phase 3 SINUS trials were double-blind and placebo-controlled in patients with CRSwNP. Patients remained on their asthma treatment regimens for the duration of the trial and all were treated with mometasone furoate nasal spray (MFNS). Dupilumab acts as a receptor agonist by binding to the alpha subunit of the IL-4 receptor (IL-4Rα). Through blockade of IL-4Rα, dupilumab modulates signalling of both the IL-4 and IL-13 pathways.
In SINUS-24, participants were randomised 1:1 to subcutaneous dupilumab 300 mg or placebo every 2 weeks (q2w). SINUS-52 participants were randomised 1:1:1 to 52 weeks of subcutaneous dupilumab 300 mg q2w, 52 weeks of placebo q2w, or to 24 weeks of subcutaneous dupilumab 300 mg q2w followed by 28 weeks of dupilumab 300 mg q4w. This analysis at 24 weeks pooled patients in both trials who received dupilumab 300 mg q2w (n=170) and placebo (n=258).
At 24 weeks, patients receiving dupilumab had significant improvements compared with placebo in upper airway measures including nasal peak inspiratory flow (Least Squares [LS] mean difference 46.15 L/minute; 95% CI 37.82 to 54.47) and 22-item Sino-Nasal Outcome Test score (LS mean difference -21.42; 95% CI -24.97 to -17.87) (P<0.0001 for both).
Patients receiving dupilumab also had significant improvements compared with placebo at 24 weeks in lower airway outcomes including FEV1 (LS mean difference 0.21; 95% CI 0.13 to 0.29) and the 6-item Asthma Control Questionnaire score (LS mean difference -0.82; 95% CI -0.98 to -0.67) (P<.0001 for both, see Table). Adverse events occurring in >5% of patients were nasopharyngitis, nasal polyps, headache, injection site erythema, asthma, and epistaxis; all of which occurred more frequently in patients treated with placebo. “Dupilumab was effective in improving lower airway outcome measures regardless of blood eosinophil levels,” Dr Maspero shared in his presentation.
Table: Change from baseline at week 24 in upper and lower airway outcome measures [1]
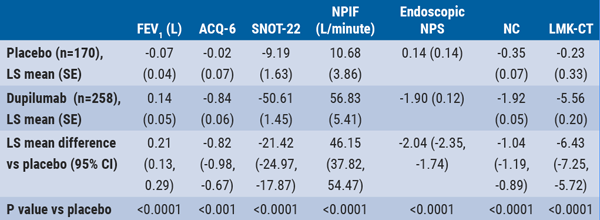 Clinically meaningful changes in scores for the 6-item Asthma Control Questionnaire score (ACQ-6), the 22-item Sino-Nasal Outcome Test score (SNOT-22), nasal peak inspiratory flow (NPIF), nasal polyp score (NPS), nasal congestion (NC), and Lund-MacKay computed tomography (LMK-CT). CI, confidence interval; FEV1, forced expiratory volume in 1 second; LS, least squares; SE, standard error.
Clinically meaningful changes in scores for the 6-item Asthma Control Questionnaire score (ACQ-6), the 22-item Sino-Nasal Outcome Test score (SNOT-22), nasal peak inspiratory flow (NPIF), nasal polyp score (NPS), nasal congestion (NC), and Lund-MacKay computed tomography (LMK-CT). CI, confidence interval; FEV1, forced expiratory volume in 1 second; LS, least squares; SE, standard error.
- Laidlaw T, et al. A7536, ATS 2019, 17-22 May, Dallas, Texas, USA.
Posted on
« CRISPR/Cas9 genome editing therapy of hereditary pulmonary alveolar proteinosis Next Article
Bacterial pneumonia predicts ongoing lung problems in infants hospitalised for acute respiratory failure »
Table of Contents: ATS 2019
Featured articles
Letter from the Editor
Interview with Prof. Christian Bergmann
Treatable Traits in Chronic Inflammatory Airway Disease: Back to Basics
Treatable traits in chronic inflammatory airway disease: back to basics
Critical Care Medicine
Distinguishing between 4 different subtypes of sepsis sets the stage for individualised treatment
Stem cell therapy in acute respiratory distress syndrome improves 28-day mortality
SPICE III trial: Early sedation with dexmedetomidine in critically ill patients
SAATELLITE trial: Suvratoxumab prevents ventilator-associated Staphylococcus Aureus pneumonia in intensive care unit patients
Sleep Medicine
Million-patient study reveals gaps in long-term adherence among various sub-populations
Sleep apnoea severity has a non-linear relationship with acute myocardial infarction risk
Obstructive sleep apnoea affects morning spatial navigational memory processing in asymptomatic older individuals
Pulmonary Vascular Disease and Interstitial Lung Disease
Nintedanib reduces lung function decline in systemic sclerosis-associated ILD
Pulmonary arterial hypertension: early treatment with selexipag most effective
Long-term safety and efficacy of recombinant human pentraxin-2 in patients with idiopathic pulmonary fibrosis
Infection
Dupilumab improves outcomes in patients with severe chronic rhinosinusitis with nasal polyps and comorbid asthma
Durability of culture conversion in patients receiving ALIS for treatment-refractory MAC lung disease
E-cigarette use disrupts normal immune response to viral infections, particularly in women
Paediatric Pulmonary Medicine
Bacterial pneumonia predicts ongoing lung problems in infants hospitalised for acute respiratory failure
Aspergillus and early cystic fibrosis lung disease: does it need to be treated?
COPD
CORTICO-COP trial: eosinophil-guided therapy reduces systemic corticosteroid exposure
A randomised controlled trial of a smoking cessation smartphone application
Benralizumab does not ameliorate COPD exacerbations (GALATHEA/TERRANOVA trials)
Aclidinium bromide delays COPD exacerbation without increased MACE risk
Bench-to-Bedside (Pre-Clinical)
Human lung organoids to study foetal RSV infection
CRISPR/Cas9 genome editing therapy of hereditary pulmonary alveolar proteinosis
Cilia diagnostics in primary ciliary dyskinesia
Tuberous sclerosis complex 2 may be a novel target in pulmonary arterial hypertension therapy
Related Articles
Sodium intake and blood pressure: new insights

© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

