Dr Pradeesh Sivapalan (University of Copenhagen, Denmark) presented the research in a late-breaking clinical trial session, noting that the work was being simultaneously published in The Lancet Respiratory Medicine [1,2]. Corticosteroids are frequently used in the treatment of acute COPD exacerbations. However, the use of systemic corticosteroids is not without risks. As such, strategies to limit systemic corticosteroid exposure are needed. In this multicentre randomised controlled trial, 318 patients age 40 years and older with known airflow limitation (post-bronchodilator FEV1/FVC 0.7) and established COPD were randomised to either eosinophil-guided therapy or standard therapy with systemic corticosteroids to determine whether an algorithm using blood eosinophil counts could safely reduce systemic corticosteroid exposure in patients admitted for the treatment of acute COPD exacerbation.
All patients received 80 mg of intravenous methylprednisolone on the first day of admission. From the second day onwards, patients assigned to the eosinophil-guided group were given 37.5 mg of oral prednisolone daily, up to a maximum of 4 days, when their blood eosinophil count was at least 0.3 x 109 cells/L. On days where the eosinophil count did not meet this threshold, prednisolone was not administered. If a patient was discharged during the treatment period, a discharge prescription for the remaining days within a 5-day period was based on the last measured eosinophil count. The standard therapy group received 37.5 mg of oral prednisolone daily from the second day for 4 days. The primary outcome measure was the number of days alive and out of hospital within 14 days after recruitment.
Researchers found that there was no between-group difference for days alive and out of hospital within 14 days after recruitment (P=0.34). Treatment failure at 30 days was also comparable between groups (P=0.90). At 30 days of follow-up, 6% of patients in the eosinophil-guided group and 4% of patients in the control group had died (P=0.43). However, the median duration of systemic corticosteroid therapy was lower in the intervention group (P<0.0001, see Figure). This study, therefore, shows that although eosinophil-guided therapy does reduce systemic corticosteroid exposure, this strategy may not confer a benefit in terms of the number of days alive and out of hospital. Further studies are needed in assessing eosinophil-guided therapy and other long-term outcomes.
Figure: (A) Proportions of patients on OCS; (B) Mean duration of OCS use. Data based on [2]
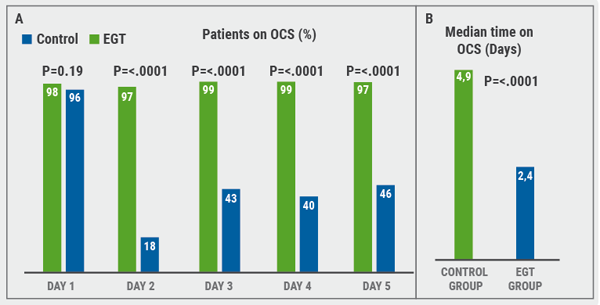
EGT, eosinophil-guided therapy; OCS, oral corticosteroids. Figure kindly provided by Dr Sivapalan.
- Sivapalan P, et al. A7352, ATS 2019, 17-22 May, Dallas, Texas, USA.
- Sivapalan P, et al. Lancet Respir Med. 2019 May 17.
Posted on
Previous Article
« Letter from the Editor Next Article
Stem cell therapy in acute respiratory distress syndrome improves 28-day mortality »
« Letter from the Editor Next Article
Stem cell therapy in acute respiratory distress syndrome improves 28-day mortality »
Table of Contents: ATS 2019
Featured articles
Letter from the Editor
Interview with Prof. Christian Bergmann
Treatable Traits in Chronic Inflammatory Airway Disease: Back to Basics
Treatable traits in chronic inflammatory airway disease: back to basics
Critical Care Medicine
Distinguishing between 4 different subtypes of sepsis sets the stage for individualised treatment
Stem cell therapy in acute respiratory distress syndrome improves 28-day mortality
SPICE III trial: Early sedation with dexmedetomidine in critically ill patients
SAATELLITE trial: Suvratoxumab prevents ventilator-associated Staphylococcus Aureus pneumonia in intensive care unit patients
Sleep Medicine
Million-patient study reveals gaps in long-term adherence among various sub-populations
Sleep apnoea severity has a non-linear relationship with acute myocardial infarction risk
Obstructive sleep apnoea affects morning spatial navigational memory processing in asymptomatic older individuals
Pulmonary Vascular Disease and Interstitial Lung Disease
Nintedanib reduces lung function decline in systemic sclerosis-associated ILD
Pulmonary arterial hypertension: early treatment with selexipag most effective
Long-term safety and efficacy of recombinant human pentraxin-2 in patients with idiopathic pulmonary fibrosis
Infection
Dupilumab improves outcomes in patients with severe chronic rhinosinusitis with nasal polyps and comorbid asthma
Durability of culture conversion in patients receiving ALIS for treatment-refractory MAC lung disease
E-cigarette use disrupts normal immune response to viral infections, particularly in women
Paediatric Pulmonary Medicine
Bacterial pneumonia predicts ongoing lung problems in infants hospitalised for acute respiratory failure
Aspergillus and early cystic fibrosis lung disease: does it need to be treated?
COPD
CORTICO-COP trial: eosinophil-guided therapy reduces systemic corticosteroid exposure
A randomised controlled trial of a smoking cessation smartphone application
Benralizumab does not ameliorate COPD exacerbations (GALATHEA/TERRANOVA trials)
Aclidinium bromide delays COPD exacerbation without increased MACE risk
Bench-to-Bedside (Pre-Clinical)
Human lung organoids to study foetal RSV infection
CRISPR/Cas9 genome editing therapy of hereditary pulmonary alveolar proteinosis
Cilia diagnostics in primary ciliary dyskinesia
Tuberous sclerosis complex 2 may be a novel target in pulmonary arterial hypertension therapy
Related Articles
November 9, 2021
COPD symptoms and lung function related to sleep quality

November 24, 2018
Letter from The Editor
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

