She started by briefly mentioning an innovative botulinum topical gel (RT001), a substance that first appeared promising in phase 1 and 2 studies, but which failed to meet clinical endpoints during phase 3 clinical trials. The development plan for RT001 was discontinued and it was decided to focus on the injectable botulinum toxin A version RT002. Agents with a prolonged effect in chronic conditions, fewer injection series over time, a reduced waning effect between injections, and improved patient satisfaction might provide a prolonged optimal benefit. A safety and tolerability study in cervical dystonia has been completed which revealed that the investigated agent was well tolerated at both doses [1].
Prof. Comella also talked about botulinum toxin type A2, which may have a higher potency to cleave SNAP-25 cell molecules, yet with only pilot clinical data.
Moreover, she mentioned the clinical development of botulinum toxin serotype E with a potential faster onset of action within one day and a faster waning effect (two to four weeks) than botulinum toxin A. Potential benefits of rapid onset and short duration would allow a rapid relief of symptoms, may contribute to healing after injury, and reduce scar formation by reducing muscle activity. It would further allow pre-testing patterns of injection, prior to injection of longer duration botulinum toxins as well as short-term "boosting" effects in suboptimal results with longer acting botulinum toxins without an overlap at the next injection visit.
She further talked about future indications for botulinum toxin such as atrial tachycardia and atrial fibrillation [2], hip osteoarthritis [3], leg cramps in diabetic neuropathy [4], neuropathic pain (trigeminal neuralgia, post herpetic neuralgia, diabetic neuropathy, post-stroke pain, spinal cord injury), complex regional pain syndrome [5,6], major depression [7] and essential tremor [8,9] (Table 1).
Table 1: Clinical applications of botulinum toxin [10-11]
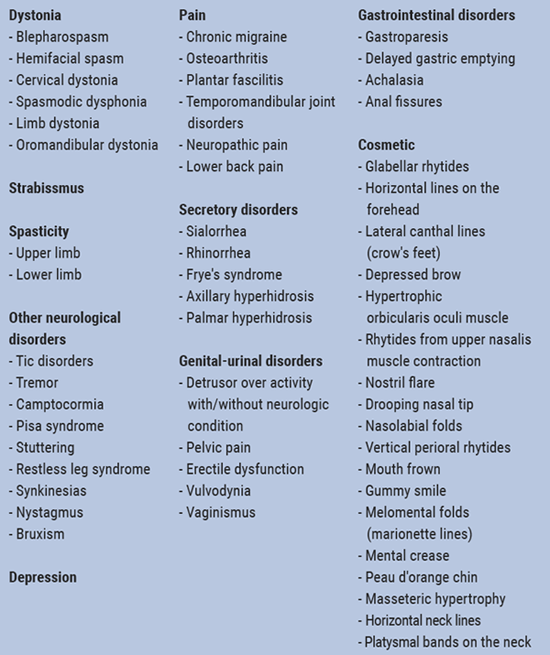
- Jankovic J, et al. Mov Disord Clin Pract. 2018 Apr 26;5(3):273-282.
- Romanov A, et al. Heart Rhythm. 2019 Feb;16(2):172-177.
- Eleopra R, et al. Toxins. 2018 Oct 31;10(11).
- Restivo DA, et al. Ann Neurol, 2018 Nov;84(5):674-682.
- Park J, Chung ME. Toxins. 2018 Jun 1;10(6).
- Park J, Park HJ. Toxins. 2017 Aug 24;9(9).
- Magid M, et al. J Clin Psychiatry. 2014 Aug;75(8):837-44.
- Samotus O, et al. Can J Neurol Sci. 2018 Jan;45(1):11-22.
- Niemann N, Jankovic J. Toxins. 2018 Jul 19;10(7).
- Samizadeh S, De Boulle K. Clin Cosmet Investig Dermatol, 2018 May 30;11:273-287.
- Fonfria E, et al. Toxins. 2018 May 18;10(5).
Posted on
Previous Article
« Utility of botulinum toxin in Parkinson’s disease beyond sialorrhea Next Article
Pain and sensory mechanisms »
« Utility of botulinum toxin in Parkinson’s disease beyond sialorrhea Next Article
Pain and sensory mechanisms »
Table of Contents: TOXINS 2019
Featured articles
Pain
Pain subsides before effect on muscles become apparent
Migraine
Central and peripheral mechanisms in migraine
Predictors of response
Spasticity
Why treat spasticity?
ASPIRE: High patient and clinician satisfaction
Cervical Dystonia
Anterocollis posture and deep cervical muscle injections
Daxibotulinum toxin in isolated cervical dystonia
Parkinson
Utility of botulinum toxin in Parkinson’s disease beyond sialorrhea
New Versions of Botulinum Toxins
New Versions of Botulinum Toxins
Related Articles
March 15, 2019
Daxibotulinum toxin in isolated cervical dystonia
March 15, 2019
Why treat spasticity?

March 15, 2019
Pain subsides before effect on muscles become apparent
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

