Potential mechanism of muscular pain
"One of the suggested mechanisms of muscular pain is that increased muscle tension is responsible for ischaemia due to compression of muscle and blood vessels. This, in turn, increases the release of a number of neuromodulators, including bradykinin and ATP, which might sensitise nociceptors," Prof. Attal explained.
According to Prof. Attal, one characteristic molecular feature accounts for the classical effects of botulinum toxin on dystonia and on muscle pain, and that is the internalisation and fixation on SNAP-25 and inhibition of acetylcholine exocytosis. Furthermore, pronounced analgesic effects appear even before the drug's effect on muscle contraction [1].
Directly reducing nociceptive activity
She speculated that beyond its well-known effect on muscle contraction, botulinum toxin might also exert a direct anti-nociceptive effect that is completely independent from its well-known classical effect on muscle tone. Many animal studies testing this hypothesis have started to emerge. In the formalin test model, peripheral injection of botulinum toxin in animals improved nociceptor behaviour in a dose-dependent manner, suggesting that this indeed is an anti-nociceptor effect [2]. Peripheral injections of botulinum toxin in further animal models suggested that botulinum toxin might have an effect on neurogenic inflammation and may be effective in many pain conditions. Another experimental pain model, mimicking what happens in chronic pain states, revealed its effect on TRPV1 receptors, which may explain why botulinum toxin A is able to reduce capsaicin-evoked pain in human skin in healthy volunteers. Botulinum toxin-pretreated subjects experienced reduced pain when capsaicin was applied [3-5].
Neuropathic pain – unmet medical need
"All this data from animal and human observations prompted us some years ago to explore the potential activity of botulinum toxin in peripheral neuropathic pain. Several conditions are associated with peripheral neuropathic pain, for example, post-herpetic neuralgia, polyneuropathy, postsurgical neuropathic pain, and radicular pain. Recent clinical trials and a recent meta-analysis have shown that the number of responders to current treatment options compared with placebo is relatively low”. Prof. Attal emphasised that these conditions are very difficult to treat and this is why she urged that other therapeutic options are needed.
In patients with peripheral neuropathic pain, a first randomised, double-blind, placebo-controlled trial was published in 2008 [6]. Patients with peripheral neuropathic pain were selected on the basis of allodynia in the painful area. The neurotoxin was injected subcutaneously or deep intra-dermally. The results of this pilot study revealed indeed some efficacy of botulinum toxin vs placebo. The effect set in approximately 7 days after the initial set of injections and effects were remained even after 24 weeks [6]. Based on a meta-analysis of small but convincing clinical studies published in 2015 [7], "we proposed to recommend botulinum toxin A as a last choice; however, to recommend it with some limitations" (third-line recommendation for patients with peripheral neuropathic pain with a local pain generator).
In patients with post-herpetic neuralgia, the neurotoxin also demonstrated some efficacy compared with saline and placebo, its efficacy also lasting up to 3 months (VAS score) [8]. However, for treatment of patients with trigeminal neuralgia, botulinum toxin A is not yet recommended by scientific societies [9].
Repeated injections
Since data on efficacy and safety of repeated administrations of botulinum toxin A were lacking, Prof. Attal designed another double-blind, randomised trial [10]. She pointed out that repeated injections are essential when treating chronic pain patients. She added: "We wanted to determine whether a second injection could have the same or even better efficacy." Thus safety and efficacy of repeated injections of botulinum toxin A (2 successive administrations within 12 weeks) was evaluated in patients with moderate-to-severe peripheral neuropathic pain (limited to a maximum area of 240 cm2 due to dose limitations of 300 units of botulinum toxin A in this study, to avoid any potential side effects). A total of 152 patients were screened and 68 patients with moderate to severe peripheral neuropathic pain were randomised. In three centres, in Paris, Limoges, and Sao Paulo, the patients received multiple subcutaneous injections of botulinum toxin A into the painful area. Clinical assessment was performed via specific validated neuropathic pain questionnaires and quantitative sensory testing. At baseline and at one month, intradermal fibre density (IENFD) was evaluated at the painful and the control side by skin punch biopsy. At the same timepoints, levels of calcitonin gene-related peptide (CGRP) and substance P (SP) - substances involved in neurogenic inflammation - were evaluated at the painful side.
Results showed that 2 successive administrations of botulinum toxin A, each consisting of several injections, significantly decreased pain intensity over a period of 24 weeks compared with placebo (P<0.0001). A sustained analgesic effect in peripheral neuropathic pain was reported (Figure 1). Moreover, analgesic efficacy was enhanced by a second administration 12 weeks after the first. Almost a quarter of the patients who only demonstrated little improvement after the first administration, responded favourably to the second administration. This finding suggests that 2 administrations of botulinum toxin A may be essential before concluding that a patient with neuropathic pain does not respond to the therapy.
Evaluation of the questionnaires revealed that botulinum toxin A had a very significant and prolonged effect on paroxysmal pain; not only on pain intensity but also on number of paroxysmal pain (Figure 2). Botulinum toxin A significantly reduced allodynia (P=0.0002) and relieved hyperalgesia to mechanical stimuli on the painful side compared with placebo (P=0.03). Efficacy on average pain intensity during the study duration was greater in participants with allodynia than in those without allodynia at study entry. Neuropeptide concentrations (CGRP, SP) in skin punch biopsies were similar in both treatment arms and were not modified by botulinum toxin A injections [10].
Figure 1 Effects of botulinum toxin A vs placebo on the primary study endpoint, average pain intensity over a 24-week period of time [12]

Figure 2 Effects of botulinum toxin A on paroxysmal pain (Neuropathic Pain Symtom Inventory) vs placebo [12]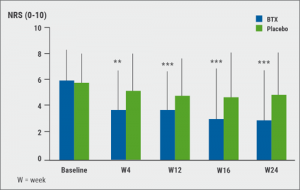
When searching for predictors of response to botulinum toxin A, it was observed that patients who seemed to be the best responders were those who had very little evidence for thermal deficit at baseline, Prof. Attal explained. Moreover, presence, intensity, and severity of (mechanical) allodynia at baseline was a predictor of response to botulinum toxin A. Prof. Attal added that "this was also confirmed by a more objective marker of fibre loss which is skin punch biopsy." The less impairment of intra-epidermal fibre density at baseline and the more preserved nociceptive function, the better the response to botulinum toxin A [10].
Take-home message
Prof. Attal concluded that there is strong experimental rationale for the use of botulinum toxin A as an analgesic. Moreover, there is growing clinical evidence for its use in neuropathic pain. She emphasised that the safety profile is excellent and that its mechanisms of action might be central. She wondered, "who could be the ideal patient for botulinum toxin?" According to her, ideal candidates would be "patients with peripheral, maybe central, neuropathic pain, or trigeminal neuralgia, irresponsive to conventional therapy. This is off label! Preserved nociceptive function and mechanical allodynia seem to be a very good predictor for response, at least from our study. Patients also with paroxysmal pain are most likely very good responders for this drug."
- Tsui JK, et al. Can J Neurol Sci. 1985 Nov;12(4):314-6.
- Cui M, et al. Pain. 2004 Jan;107(1-2):125-33.
- Morenilla-Palao C, et al. J Biol Chem. 2004 Jun 11;279(24):25665-72.
- Tugnoli V, et al. Pain, 2007 Jul;130(1-2):76-83.
- Gazerani P, et al. Pain. 2006 Jun;122(3):315-25.
- Ranoux D, et al. Ann Neurol. 2008 Sep;64(3):274-83.
- Finnerup NB, et al. Lancet Neurol. 2015 Feb;14(2):162-73.
- Xiao L, et al. Pain Med. 2010 Dec;11(12):1827-33.
- Cruccu G, et al. Neurology. 2016 Jul 12;87(2):220-8.
- Attal N, et al. Lancet Neurol. 2016 May;15(6):555-65.
Posted on
Previous Article
« Letter from the ESC Congress Programme Committee Chairs Next Article
Predictors of response »
« Letter from the ESC Congress Programme Committee Chairs Next Article
Predictors of response »
Table of Contents: TOXINS 2019
Featured articles
Pain
Pain subsides before effect on muscles become apparent
Migraine
Central and peripheral mechanisms in migraine
Predictors of response
Spasticity
Why treat spasticity?
ASPIRE: High patient and clinician satisfaction
Cervical Dystonia
Anterocollis posture and deep cervical muscle injections
Daxibotulinum toxin in isolated cervical dystonia
Parkinson
Utility of botulinum toxin in Parkinson’s disease beyond sialorrhea
New Versions of Botulinum Toxins
New Versions of Botulinum Toxins
Related Articles

March 4, 2019
Letter from the Editor
March 15, 2019
Central and peripheral mechanisms in migraine
March 15, 2019
Lessons learned from triptan therapy
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

