These 1-year interim data from the ASPIRE study, presented by Dr Francisco, continue to support safety and effectiveness of onabotulinum toxin A treatment for spasticity in clinical practice. Despite variation in dosing, patient and physician satisfaction across aetiologies was high.
Posted on
Previous Article
« Predictors of response Next Article
Daxibotulinum toxin in isolated cervical dystonia »
« Predictors of response Next Article
Daxibotulinum toxin in isolated cervical dystonia »
Table of Contents: TOXINS 2019
Featured articles
Pain
Pain subsides before effect on muscles become apparent
Migraine
Central and peripheral mechanisms in migraine
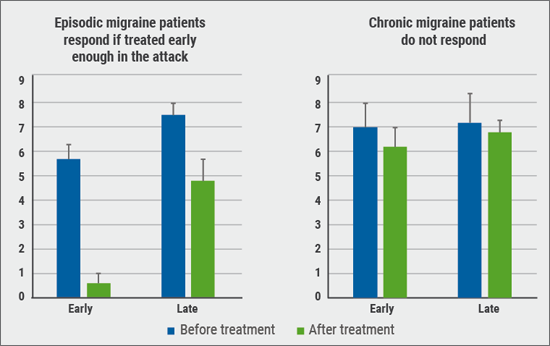
Predictors of response
Spasticity
Why treat spasticity?
ASPIRE: High patient and clinician satisfaction
Cervical Dystonia
Anterocollis posture and deep cervical muscle injections
Daxibotulinum toxin in isolated cervical dystonia
Parkinson
Utility of botulinum toxin in Parkinson’s disease beyond sialorrhea
New Versions of Botulinum Toxins
New Versions of Botulinum Toxins
Related Articles
March 15, 2019
Predictors of response
March 15, 2019
Lessons learned from triptan therapy
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

