In the evaluation presented, 473 patients were included until December 2016: this corresponds to 264 patient years of biological and 272 years of non-biological systemic therapy [1]. Their mean age was 46.7 years, 65% were male and more than a third not only suffered from psoriasis but also from psoriatic arthritis.
Rates of serious adverse events and non-serious infection rates were not significantly different between the two treatment groups. The most reported non-serious adverse events were ineffectiveness of the drug with 15.8 % of patients treated with biologics and 16.6% in the non-biological group. Non-serious gastrointestinal side effects were significantly higher in the non-biological cohort compared to biological regimes (14.1 vs. 4.3/100 patient years; P≤0.05). All in all, serious adverse events were uncommon and without a different distribution among the two kinds of treatment.
1. Maul, JT. et al. P5975, AAD Annual Meeting, February 16–20 2018.
Posted on
Previous Article
« Living in the golden age of psoriasis and atopic dermatitis therapies Next Article
Letter from The Editor »
« Living in the golden age of psoriasis and atopic dermatitis therapies Next Article
Letter from The Editor »
Table of Contents: AAD 2018
Featured articles
Letter from The Editor
Living in the golden age of psoriasis and atopic dermatitis therapies
Late-breakers
IL-17C inhibition in AD and new oral treatments
Dual JAK/SYK inhibitor and anti-IL-33 blockade
Psoriasis: Selective IL-23 blocker, analysis of VOYAGE-2, dual IL-17 inhibitor and ustekinumab
Hyperhidrosis: Soft molecule and anticholinergic towelettes
Behcet’s syndrome and hidradenitis suppurativa
Psoriasis: an update
Oral therapeutics, supersaturation and excimer laser
Psoriasis management online?
What's hot in atopic dermatitis
AD sleep disturbance, antihistamines and osteoporosis
New topical and systematic treatments
Acne management
Winter effect and preventing scarring
Restrictive antibiotic use and novel tetracycline
Alopecia Areata
Melanoma
Melanoma incidence continues to rise in Europe
Lesions in paediatric patients and possible correlation with coffee drinking
CNNs and targeted combination therapy
Pearls of the posters
Improvement in impact of genital psoriasis on sexual activity with use of ixekizumab
Intralesional cryosurgery and itching in psoriasis
Related Articles
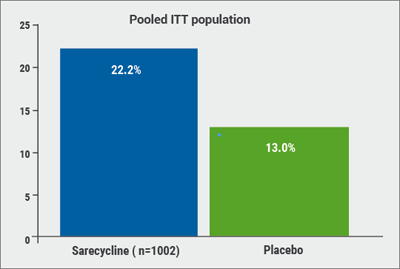
December 20, 2018
Restrictive antibiotic use and novel tetracycline
January 31, 2019
Letter from The Editor
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

