“Malignant melanoma is primarily diagnosed visually, beginning with an initial clinical screening,” said Dr Allan Halpern [9]. This step is then followed by dermoscopic analysis, a biopsy and histopathological examination.
Artificial intelligence—deep convolutional neural networks (CNNs) —may help in diagnosis, according to a recent publication [10]. CNNs are feed-forward artificial neural networks and learn the filters that, in traditional algorithms, were hand-engineered. This makes them independent of prior knowledge and human effort.
The working group at Stanford University in California trained this system with a dataset of 129,450 clinical images consisting of 2,032 different diseases (two orders of magnitude larger than previous datasets). The CNN was then tested against 21 board-certified dermatologists on biopsy-proven clinical images.
The result was astonishing: the CNN achieved a performance on par with all tested experts, and classified melanoma with a level of competence comparable to the dermatologists. Even regarding the biopsy decision, the dermatologists did not outperform the CNN. According to the authors of the study, deep neural networks deployed on mobile devices can potentially extend the reach of dermatologists outside the clinic. This technique could provide low-cost universal access to vital diagnostic care [10].
“The digital ability to monitor lesions over time is going to be the state of the art in the future: the technology is already there,” said Dr Halpern.
Survival benefit of targeted combination therapy
New data are also available about therapy of metastatic melanoma. A trial published in 2017 confirmed that patients with BRAF600-mutant metastatic melanoma have a persistent overall and progression-free survival benefit when they are treated with the combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib [11]. Overall survival was 30% at four years and 28% at five. Survival was higher among patients with normal baseline LDH levels. This five-year analysis represents the longest follow-up to date with BRAF + MEK inhibitor combination therapy in BRAF V600-mutant melanoma. It shows that this therapy really elicits durable plateaus of long-term overall and progression-free survival that can last more than five years in some patients (see Figure) [11].
Figure: Overall survival of patients with malignant melanoma in the intention-to-treat-population [11]
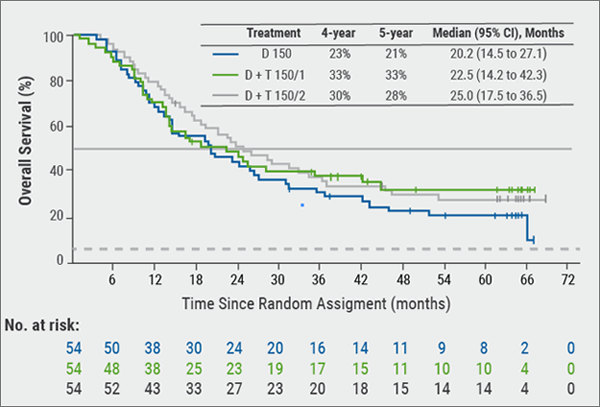
D = Dabrafenib; D + T 150/1 = dabrafenib 150 mg twice a day plus trametinib 1 mg once daily: D + T 150/2 = dabrafenib 150 mg twice a day plus trametinib, 2 mg once a day.
However, treating physicians have to deal with the different toxicities of this regime. According to Dr Halpern, checkpoint inhibitors are also relevant in the adjuvant setting: in December 2017, FDA granted regular approval to the immune checkpoint inhibitor nivolumab for patients with melanoma involving lymph nodes or in patients with metastatic disease who have undergone complete resection [12]. Nivolumab was previously approved only for the treatment of patients with unresectable or metastatic melanoma. Approval was based on improvement in recurrence-free survival in the CHECKMATE-238 trial that included 906 patients with completely resected, Stage III B/C or Stage IV melanoma [13]. Patients were randomly allocated (1:1) to receive nivolumab 3 mg/kg every two weeks or ipilimumab 10 mg/kg every three weeks for four doses, then every 12 weeks beginning at Week 24 for up to one year. Patients in the nivolumab arm experienced significantly fewer recurrences/deaths compared with the ipilimumab arm (34% vs. 45.5%; P<0.0001) [13].
“The problem with immunotherapy is the toxicity like hypophysitis, but rash is also a big piece of it,” concluded Dr Halpern.
9. Halpern, A. oral presentation S048, AAD Annual Meeting, February 16–20 2018
10. Esteva, A. et al. Nature. 2017; 542:115–18
11. Long, GV. et al. J Clin Oncol 2018;36:667–73.
12. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm590004.htm, last accessed March 12.
13. Weber, J. et al. N Engl J Med 2017:377:1824–35.
Posted on
Previous Article
« Hyperhidrosis: Soft molecule and anticholinergic towelettes Next Article
Psoriasis: Selective IL-23 blocker, analysis of VOYAGE-2, dual IL-17 inhibitor and ustekinumab »
« Hyperhidrosis: Soft molecule and anticholinergic towelettes Next Article
Psoriasis: Selective IL-23 blocker, analysis of VOYAGE-2, dual IL-17 inhibitor and ustekinumab »
Table of Contents: AAD 2018
Featured articles
Letter from The Editor
Living in the golden age of psoriasis and atopic dermatitis therapies
Late-breakers
IL-17C inhibition in AD and new oral treatments
Dual JAK/SYK inhibitor and anti-IL-33 blockade
Psoriasis: Selective IL-23 blocker, analysis of VOYAGE-2, dual IL-17 inhibitor and ustekinumab
Hyperhidrosis: Soft molecule and anticholinergic towelettes
Behcet’s syndrome and hidradenitis suppurativa
Psoriasis: an update
Oral therapeutics, supersaturation and excimer laser
Psoriasis management online?
What's hot in atopic dermatitis
AD sleep disturbance, antihistamines and osteoporosis
New topical and systematic treatments
Acne management
Winter effect and preventing scarring
Restrictive antibiotic use and novel tetracycline
Alopecia Areata
Melanoma
Melanoma incidence continues to rise in Europe
Lesions in paediatric patients and possible correlation with coffee drinking
CNNs and targeted combination therapy
Pearls of the posters
Improvement in impact of genital psoriasis on sexual activity with use of ixekizumab
Intralesional cryosurgery and itching in psoriasis
Related Articles

December 21, 2018
Living in the golden age of psoriasis and atopic dermatitis therapies
December 20, 2018
AD sleep disturbance, antihistamines and osteoporosis
December 20, 2018
CNNs and targeted combination therapy
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

