A substantial proportion of patients experiences increased problems after sexual activity. In an observational, multicentre study of 354 participants, 87% reported itch, 39% pain, 42% dyspareunia, 32% a worsening of their genital psoriasis after intercourse, and 43% a decreased frequency of intercourse [5].
The current Phase 3b randomised controlled trial by Dr Jennifer Cather of the Aesthetics Center in Dallas and her colleagues therefore investigated whether treatment of genital psoriasis with the IL-17 blocker ixekizumab has a positive influence on the sexual activity of patients [6]. 149 participants were randomised to receive either a placebo over 12 weeks or 80 mg of ixekizumab subcutaneous every two weeks after a starting dose of 160 mg. The study subjects all had moderate-to-severe genital psoriasis.
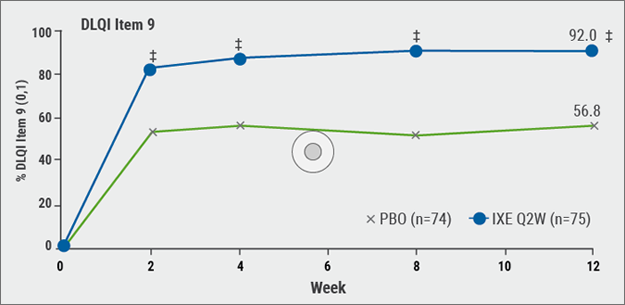
Its influence on sexual activity was evaluated by extracting specific questions from the Genital Psoriasis Sexual Frequency Questionnaire. At 12 weeks, 92% of patients treated with ixekizumab compared to 56.8% of patients treated with placebo reported no (0) or little (1) sexual difficulties caused by skin symptoms (P<0.001; see Figure). 78.4% of patients treated with ixekizumab, compared to 21.4% of patients treated with placebo (P<0.001), reported the frequency of sexual activity was either never (0) or rarely (1) limited by genital psoriasis. Ixekizumab was superior to placebo as early as Week 1 regarding the limitations on the frequency of sexual activity due to genital psoriasis (P<0.05) and Week 2 for the sexual difficulties caused by skin symptoms (P<0.001). A greater number of patients under ixekizumab stated considerably more often that they never or rarely avoided sexual activity owing to their genital psoriasis at Week 12 (76.7% IXE vs. 25.7 placebo, P<0.001).
Figure: Proportion of patients treated either with ixekizumab or placebo whose skin did not cause any sexual difficulties [6].
The ixekizumab group also experienced a markedly reduced worsening of local psoriasis symptoms during or following sexual activity at Weeks 2, 4 and 8. The most common (greater than 4%) adverse events observed in patients treated with ixekizumab in this study were upper respiratory tract infections, injection-site reactions, headache, oropharyngeal pain and pruritus. The safety outcomes were consistent with the overall safety profile of the agent in previous clinical trials.
2. Ryan. et al. J Am Acad Dermatol. 2015;72:978–83.
3. Meeuwis. et al. Acta Derm Venereol 2015;95:211–6.
4. Czuczwar, P. et al. Ginekol Pol 2016;87:717–721.
5. Ryan. et al. J Am Acad Dermatol. 2015;72:978–83.
6. Cather, JC. et al. P5935, AAD Annual Meeting, February 16–20 2018.
Posted on
Previous Article
« Intralesional cryosurgery and itching in psoriasis Next Article
Living in the golden age of psoriasis and atopic dermatitis therapies »
« Intralesional cryosurgery and itching in psoriasis Next Article
Living in the golden age of psoriasis and atopic dermatitis therapies »
Table of Contents: AAD 2018
Featured articles
Letter from The Editor
Living in the golden age of psoriasis and atopic dermatitis therapies
Late-breakers
IL-17C inhibition in AD and new oral treatments
Dual JAK/SYK inhibitor and anti-IL-33 blockade
Psoriasis: Selective IL-23 blocker, analysis of VOYAGE-2, dual IL-17 inhibitor and ustekinumab
Hyperhidrosis: Soft molecule and anticholinergic towelettes
Behcet’s syndrome and hidradenitis suppurativa
Psoriasis: an update
Oral therapeutics, supersaturation and excimer laser
Psoriasis management online?
What's hot in atopic dermatitis
AD sleep disturbance, antihistamines and osteoporosis
New topical and systematic treatments
Acne management
Winter effect and preventing scarring
Restrictive antibiotic use and novel tetracycline
Alopecia Areata
Melanoma
Melanoma incidence continues to rise in Europe
Lesions in paediatric patients and possible correlation with coffee drinking
CNNs and targeted combination therapy
Pearls of the posters
Improvement in impact of genital psoriasis on sexual activity with use of ixekizumab
Intralesional cryosurgery and itching in psoriasis
Related Articles

December 20, 2018
Oral therapeutics, supersaturation and excimer laser
December 20, 2018
Melanoma incidence continues to rise in Europe
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

