Hyperhidrosis has an impact on QoL comparable to, or greater than, psoriasis or eczema [12]. According to the International Hyperhidrosis Society, sweat production of >100 mL/5 min. in males, and >50 mL/5 min. in females is defined as hyperhidrosis. A new gel with a soft molecule was shown to be effective in reducing hyperhidrosis after a treatment period of only eight days [13].
“The anticholinergic drug sofpironium bromide is a soft molecule, which means that it is easily broken down and rapidly metabolised in the bloodstream” explained Dr Stacey Smith, a dermatologist in Encinitas, California. It inhibits acetylcholine-driven sympathetic and parasympathetic actions on various exocrine glands, including sweat glands (see Figure).
Figure: Mode of action of Acetylcholine receptor antagonists and other treatment modes for hyperhidrosis.

Ach = acetylcholine, mAChR = muscarinic acetylcholine receptor.
This new molecular entity has the advantage of a better local therapeutic effect with fewer systemic side effects. The efficacy and safety of this agent was assessed in a dose finding study, where a topically applied gel with three doses of sofpironium bromide was compared against a vehicle gel in 227 subjects with primary axillary hyperhidrosis.
“We did not only a sophisticated questionnaire but also a combined axillary gravimetric sweat production test. To be successful in hyperhidrosis, you must do well in both,” said Dr Smith.
At baseline, all subjects had scores of ≥3 (scale, 0-4) in the Hyperhidrosis Disease Severity Measure-Axillary (HDSM-Ax), a validated patient-reported outcome measure for hyperhidrosis and a combined axillary gravimetric sweat production (GSP) of ≥150 mg/5 min. A one-point improvement in the HDSM-Ax has been established as being clinically meaningful.
After 42 days of once-daily application to the axillae, patients treated with sofpironium bromide gel (5% and 15%) had statistically-significant higher response rates as measured by the HDSM-Ax from Day 8 until the end of therapy. In addition, sofpironium bromide-treated subjects demonstrated statistically significant reductions in GSP. The gel was also effective in a combined responder analysis. Treatment success was seen as both a HDSM-Ax improvement of at least one point and a ≥ 50% reduction in GSP. Nearly 60% of patients treated with the gel gained treatment success.
"Hyperhidrosis significantly impacts the social, occupational and emotional well-being of those affected, and there are currently very limited therapeutic options," said Dr Smith. "I am excited by the prospect of sofpironium bromide, to offer my patients a well-tolerated, effective and convenient first-line treatment option."
The gel was well-tolerated at all doses, with side effects that were transient and primarily mild-to-moderate in severity. The most common anticholinergic adverse event was dry mouth.
Anticholinergic towelettes safe and effective in paediatric hyperhidrosis
Hyperhidrosis is largely undertreated and underdiagnosed, particularly among paediatric patients [14]: “It would not be fair to exclude paediatric patients because hyperhidrosis often starts at an age of 9,” said Dr Adelaide Hebert, of the University of Texas Health Science Center in Houston, during the presentation of a subgroup analysis of the randomised controlled Phase 3 trials ATMOS-1 and ATMOS-2 [15].
The analysis showed that about 80% of paediatric patients had at least a 50% decrease in sweat production when using towelettes containing glycopyrronium tosylate, with a similar treatment benefit as adult patients (mode of action, see [Figure 4]).
The subgroup analysis focused on 44 patients from ages 9 to 16, with 25 randomised to use the towelettes with the anticholinergic agent. The remaining 19 patients were assigned wipes containing an inert vehicle substance.
After four weeks, axillary sweat production decreased by 64 mg/5 min (from a baseline mean of 146 mg/5 min). 79.9% of children assigned to the anticholinergic wipes had at least a 50% reduction in axillary sweat production, compared with 54.8% assigned to the control groups. Corresponding rates in the older patients were 74.3% and 53.0%. This was also true for the impact on QoL.
“In all endpoints, the paediatric group responded equally well as the adult group,” said Dr Hebert. “We did not just reduce the sweat, we increased QoL even in paediatric patients.” The safety profile was likewise similar in all age groups: some children had headache, some had specific anticholinergic effects such as mydriasis. Only one paediatric patient in the verum group discontinued therapy due to side effects.
“Even though the patients had some systemic cholinergic side effects, they did not want to leave the study because their QoL improved so much,” said Dr Hebert. The subgroup analysis was the first study in paediatric patients with hyperhidrosis. Topical glycopyyronium tosylate treatment provides a much-needed treatment option for paediatric patients with hyperhidrosis.
12. Naumann, M. et al. Value health 2003;6:242.
13. Smith, ST. Abstract 6761, AAD Annual Meeting, February 16–20 2018.
14. Gelbhard. et al. Pediatr Dermatol 2008;25:591–8.
15. Hebert, A. Abstract 6659, AAD Annual Meeting, February 16–20 2018.
Posted on
Previous Article
« Psoriasis management online? Next Article
CNNs and targeted combination therapy »
« Psoriasis management online? Next Article
CNNs and targeted combination therapy »
Table of Contents: AAD 2018
Featured articles
Letter from The Editor
Living in the golden age of psoriasis and atopic dermatitis therapies
Late-breakers
IL-17C inhibition in AD and new oral treatments
Dual JAK/SYK inhibitor and anti-IL-33 blockade
Psoriasis: Selective IL-23 blocker, analysis of VOYAGE-2, dual IL-17 inhibitor and ustekinumab
Hyperhidrosis: Soft molecule and anticholinergic towelettes
Behcet’s syndrome and hidradenitis suppurativa
Psoriasis: an update
Oral therapeutics, supersaturation and excimer laser
Psoriasis management online?
What's hot in atopic dermatitis
AD sleep disturbance, antihistamines and osteoporosis
New topical and systematic treatments
Acne management
Winter effect and preventing scarring
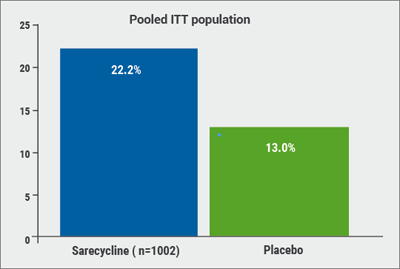
Restrictive antibiotic use and novel tetracycline
Alopecia Areata
Melanoma
Melanoma incidence continues to rise in Europe
Lesions in paediatric patients and possible correlation with coffee drinking
CNNs and targeted combination therapy
Pearls of the posters
Improvement in impact of genital psoriasis on sexual activity with use of ixekizumab
Intralesional cryosurgery and itching in psoriasis
Related Articles
December 20, 2018
Restrictive antibiotic use and novel tetracycline

December 20, 2018
Oral therapeutics, supersaturation and excimer laser

December 20, 2018
IL-17C inhibition in AD and new oral treatments
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

