https://doi.org/10.55788/3a8f3dfb
Long-term disease control is crucial in chronic disorders like PsA, especially as loss of response during treatment may be possible [1]. After demonstrating superiority over placebo at week 16 in the phase 3 BE OPTIMAL trial (NCT03895203), bimekizumab was investigated for its ability to maintain efficacy in participants with PsA [2]. The initial phase 3 cohort was randomised to bimekizumab, placebo, or adalimumab as active reference. A direct statistical comparison of the placebo arm and bimekizumab versus adalimumab could not be performed due to underpowering of the adalimumab group. The current analysis showed outcomes for responders at week 16, who were followed through a subsequent 36-week active treatment- blind period. The results were presented using non-responder imputation (NRI) as well as observed case (OC) reporting.
The baseline characteristics of the 431 participants treated with bimekizumab included a mean age of 48.5, a mean duration of PsA of 6 years, and a BMI of 29.2 kg/m2. Also, enthesitis was present in about one-third, and the mean Psoriasis Area and Severity Index (PASI) score was 8.2.
The results after 1 year revealed that out of the initial 43.9% of participants on bimekizumab who achieved an ACR50 at week 16, 86.8% (NRI) and 91.1% (OC) were able to sustain their response at week 52. In the adalimumab reference arm, with 64 responders out of 140, the corresponding rates were 79.7% and 86.4% (see Figure). PASI100 was sustained in 79.6% on bimekizumab and 71.4% on adalimumab (both NRI). Minimal disease activity, reached by 45% in both active groups at week 16, persisted in 85.6% (bimekizumab) and 82.5% (adalimumab). Of note, the simultaneous maintenance of ACR50 and PASI100 was present at week 52 in 86.7% (NRI) or 89.7% (OC) participants receiving bimekizumab, and 54.5% (NRI) or 66.7% (OC) participants on adalimumab, respectively.
Figure: Maintenance of ACR50 responses at week 52, in week 16 responders [1]
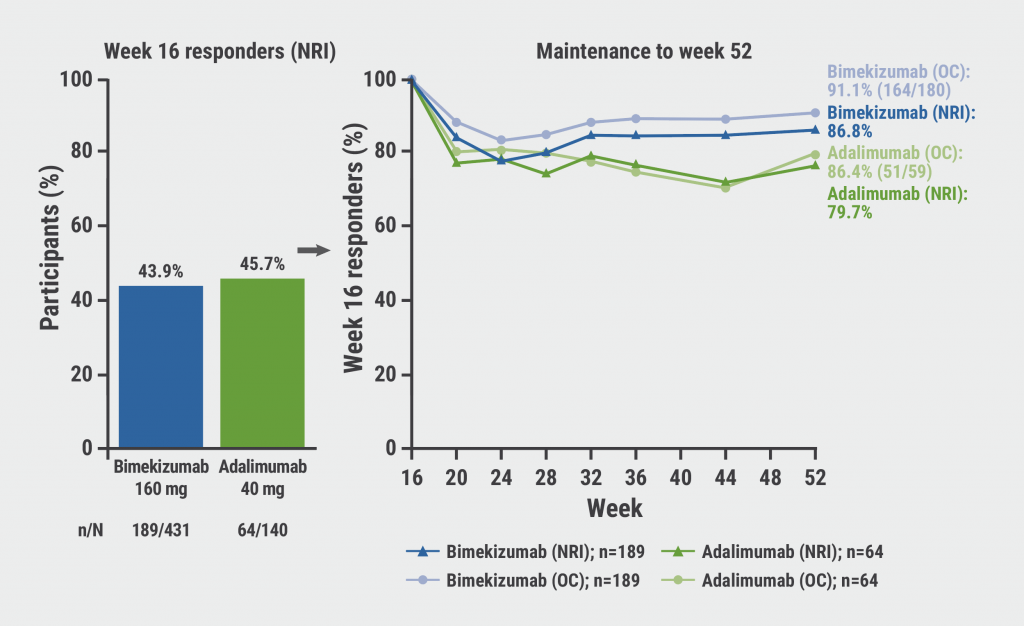
NRI, non-responder imputation; OC, observed case.
Up to year 1, a 79.1% rate of at least 1 treatment-emergent adverse event was observed in bimekizumab recipients, and 6.6% were reported as serious, which was in line with prior findings.
As comprehensive long-term disease control is important in PsA, the authors underlined that maintained robust efficacy responses were seen at week 52 in a high proportion of bimekizumab-treated initial responders from the BE OPTIMAL trial. Nearly 90% of participants persisted with a high level of response in both articular and cutaneous domains under bimekizumab, keeping in line with the previously recognised superiority of IL-17 pathway inhibition in the psoriasis space.
- Boehncke WH, Menter Am J Clin Dermatol. 2013;14:377-88.
- Tillett W, et al. Bimekizumab maintained efficacy responses through 52 weeks in biologic disease-modifying antirheumatic drug-naïve patients with psoriatic arthritis who were responders at week 16: results from BE OPTIMAL, a phase 3, active-reference study. POS1534, EULAR 2023, 31 May–3 June, Milan, Italy.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Drugs for osteoporosis: time to reach fracture risk reduction varies Next Article
Therapy with biological DMARDs shows no correlation with fracture risk in RA »
« Drugs for osteoporosis: time to reach fracture risk reduction varies Next Article
Therapy with biological DMARDs shows no correlation with fracture risk in RA »
Table of Contents: EULAR 2023
Featured articles
Late-breaking Oral Abstracts
Selective JAK1/TYK 2 inhibitor effective in patients with difficult-to-treat RA
Novel 2-drug combo improves treatment possibilities for patients with refractory gout
Dazodalibep improves dryness, fatigue, and pain in patients with Sjögren’s syndrome with a high symptom burden
COVID-19: Young adults with auto-immune diseases have different risks than their healthy counterparts
RA in 2023
Poly-refractory RA: not common, but still present
AI almost as successful as experts in predicting early RA
Worse self-management in patients with inflammatory arthritis in the presence of comorbid anxiety or depression
Disease activity-guided dose reduction may be a long-term option for stable RA
Cardiovascular safety of JAK inhibitors: reassuring results from a real-world study
Spondylarthropathies: New Developments
AxSpA: Adalimumab biosimilar equally effective as IL-17 inhibitor in hindering radiographic progression
Vascular inflammation may be characteristic of PsA
Obesity in PsA is increasingly affecting male patients
PsA patients: highest risk of developing NAFLD
What is Hot in Osteoarthritis
Lorecivivint shows long-term benefits for severe knee OA
Methotrexate lowers pain in inflammatory hand OA
Systemic Sclerosis: State of the Art
Targeted DMARDs advantageous in SSc patients with pre-capillary pulmonary hypertension
Osteoporosis: New Data
Drugs for osteoporosis: time to reach fracture risk reduction varies
Romosozumab: the new option for glucocorticoid-induced osteoporosis with high fracture risk?
Best of the Posters
Therapy with biological DMARDs shows no correlation with fracture risk in RA
Basic Science
In vitro and in vivo studies confirm the role of regulatory volume decrease
Related Articles
November 13, 2020
Osteoporosis in men often goes unnoticed, untreated
September 4, 2019
Interstitial lung disease in rheumatic diseases and systemic sclerosis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

