https://doi.org/10.55788/fc19012b
Uricase-based therapy is recommended for gout patients who have failed to achieve uric acid-lowering targets, but ADA diminish their effectiveness, leading to treatment failure rates in up to 38% of cases [1,2]. “Pegadricase is a potent pegylated uricase that converts uric acid to soluble and readily excreted allantoin,” Prof. Herbert SB Baraf (Center for Rheumatology and Bone Research, IL, USA) explained the mode of action of 1 component during the presentation [3]. However, as most uricases, it also elicits a vigorous ADA response, limiting its use as a monotherapy. Therefore, SEL-110, the second component of the novel infusion therapy, is administered 30 minutes before pegadricase. SEL-110 is an immune-tolerising nano-encapsulated rapamycin that has demonstrated dose-dependent inhibition of anti-pegadricase antibodies in previous trials.
In the phase 3 studies DISSOLVE I (NCT04513366) and DISSOLVE II (NCT04596540), participants were enrolled if they had ≥3 gout flares within 18 months before screening, ≥1 tophus, or a current diagnosis of gouty arthritis and failed to normalise serum uric acid and control symptoms with any xanthine oxidase inhibitor. They were randomised to receive a high or a low dose of the novel 2-drug combo SEL- 212 or placebo, every 28 days for a total of 6 treatments. The primary endpoint was the percentage of participants achieving serum uric acid levels <6 mg/dL for at least 80% of the sixth 28-day treatment period. “Stringent stopping rules were implemented to minimise the risk of infusion-related adverse events,” Prof. Baraf emphasised.
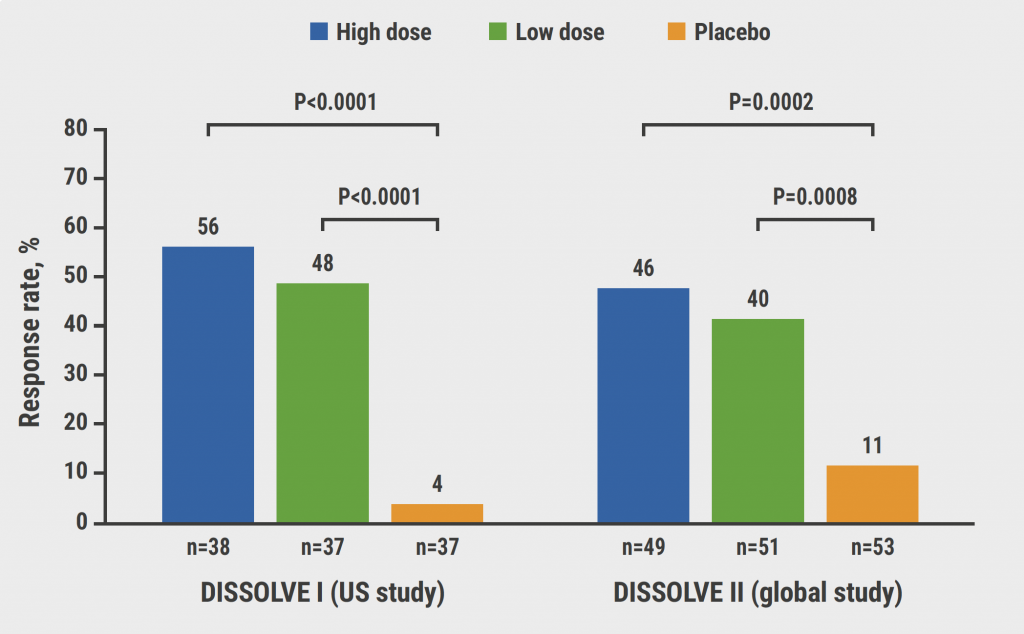
Included were 265 participants whose demographic characteristics were balanced for age, BMI, and sex, but racial imbalances existed in both studies. Gout severity was numerically greater in the 153 participants included in DISSOLVE II. The primary efficacy endpoint was met for both studies and doses (see Figure). In the DISSOLVE I study, 58% of participants in the high-dose group (n=38) and 48% in the low-dose group (n=37) responded to treatment (both P<0.0001 vs placebo). The corresponding numbers in the DISSOLVE II study were 46% in the high-dose group (P=0.0002 vs placebo) and 40% in the low-dose group (P=0.0008 vs placebo). Median change in serum uric acid levels indicates large reductions in at least half of the participants. Participants older than 50 years had similar response rates.
Treatment was relatively tolerable. The infusion reaction incidence was 3.4% in the high-dose group.
Prof. Baraf concluded that SEL-212 might potentially provide a new once-monthly uricase-based treatment option for patients with refractory gout.
Figure: Primary trial endpoint: response to SEL-212 versus placebo at week 12 [3]
- Fitzgerald JD, et Arthritis Care Res (Hoboken). 2020;72:744-60.
- Botson J, et Arthritis Rheumatol. 2023;75:293-304.
- Baraf HSB, et Safety and efficacy of SEL-212 in patients with gout refractory to conventional treatment: outcomes from two randomised, double-blind, placebo- controlled, multicenter phase III studies. LB0002, EULAR 2023, 31 May–3 June, Milan, Italy.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Dazodalibep improves dryness, fatigue, and pain in patients with Sjögren’s syndrome with a high symptom burden Next Article
Selective JAK1/TYK 2 inhibitor effective in patients with difficult-to-treat RA »
« Dazodalibep improves dryness, fatigue, and pain in patients with Sjögren’s syndrome with a high symptom burden Next Article
Selective JAK1/TYK 2 inhibitor effective in patients with difficult-to-treat RA »
Table of Contents: EULAR 2023
Featured articles
Late-breaking Oral Abstracts
Selective JAK1/TYK 2 inhibitor effective in patients with difficult-to-treat RA
Novel 2-drug combo improves treatment possibilities for patients with refractory gout
Dazodalibep improves dryness, fatigue, and pain in patients with Sjögren’s syndrome with a high symptom burden
COVID-19: Young adults with auto-immune diseases have different risks than their healthy counterparts
RA in 2023
Poly-refractory RA: not common, but still present
AI almost as successful as experts in predicting early RA
Worse self-management in patients with inflammatory arthritis in the presence of comorbid anxiety or depression
Disease activity-guided dose reduction may be a long-term option for stable RA
Cardiovascular safety of JAK inhibitors: reassuring results from a real-world study
Spondylarthropathies: New Developments
AxSpA: Adalimumab biosimilar equally effective as IL-17 inhibitor in hindering radiographic progression
Vascular inflammation may be characteristic of PsA
Obesity in PsA is increasingly affecting male patients
PsA patients: highest risk of developing NAFLD
What is Hot in Osteoarthritis
Lorecivivint shows long-term benefits for severe knee OA
Methotrexate lowers pain in inflammatory hand OA
Systemic Sclerosis: State of the Art
Targeted DMARDs advantageous in SSc patients with pre-capillary pulmonary hypertension
Osteoporosis: New Data
Drugs for osteoporosis: time to reach fracture risk reduction varies
Romosozumab: the new option for glucocorticoid-induced osteoporosis with high fracture risk?
Best of the Posters
Therapy with biological DMARDs shows no correlation with fracture risk in RA
Basic Science
In vitro and in vivo studies confirm the role of regulatory volume decrease
Related Articles
January 18, 2021
No heightened outcome risk for rheumatic patients with COVID-19
May 6, 2022
Do NSAIDs negate bone effect of bisphosphonates?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

