Enthesitis is defined as inflammation at the site of insertion of muscle or tendon in the bone. It is often located at the Achilles tendon, plantar fascia, elbows, and costochondral joints. However, it may be ubiquitous, which might be challenging to recognise. As Prof. Dennis McGonagle (University of Leeds, United Kingdom) emphasised, enthesitis has a major impact on activities of daily living in patients with psoriatic arthritis (PsA) [1]. This has been shown in the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey that examined the influence of PsA on patients' activities of daily living and unmet treatment needs [2]. It showed that enthesitis can impede walking outdoors on flat ground or bending down to pick up clothing from the floor.
In spondyloarthropathy (SpA), chronic synovitis potentially results in bone and cartilage erosion analogous to rheumatoid arthritis [3]. However, enthesitis may be the first abnormality to trigger an inflammatory response in the synovium of SpA patients. Therefore, synovitis in SpA is secondary to the liberation of proinflammatory mediators from the enthesis, resulting in entheseal erosions and periarticular abnormalities, whereas the synovitis of rheumatoid arthritis is primary [3]. Mechanical stress plays an important role in the enthesitis pathogenesis, with the disease subsequently spreading to adjacent joint structures, including the synovium and bone. Common enthesitis features also target organs of SpA beyond the joint and explain comorbid conditions like nail and skin psoriasis, inflammatory bowel disease, and uveitis. Sites of repetitive stress are, for example, nail attachments, psoriatic skin lesions over knees and elbows, lesions in the ileocecal junction, or the ciliary body. Not only are there anatomical and biomechanical similarities between the enthesis and these other structures but there is emerging evidence for similar resident immune cell populations, including IL-23R positive cells at all of these sites [4].
As Prof. McGonagle pointed out, in clinical trials, enthesitis is assessed with the Maastricht AS Enthesitis Score (MASES) including 13 sites or the Spondyloarthritis Research Consortium of Canada (SPARCC) assessing 16 sites [5]. However, in clinical trials, the Leeds Enthesitis Index (LEI), designed for use in PsA, evaluates tenderness at 6 sites: lateral epicondyles of the humerus, medial condyles of the femur, and the insertion of the Achilles tendon. “As there are only 6 sites, this is really fast to do,” Prof. McGonagle said [1].
Therapy of enthesitis: much to consider
A couple of general considerations should precede the choice of therapy. In inflammatory enthesitis, mechanical components must be identified and treated, just as inflammatory components should not be overlooked in mechanical disease. Different sites of isolated enthesitis may require different physical therapy and medication strategies, such as the use of intra-enthesis corticosteroids, or not. The location of enthesitis plays a major role in “site-specific” physical therapy strategies. Finally, patients should be informed that enthesitis is often linked to good tissue or excessive tissue repair rather than progressive joint destruction that is evident with synovitis.
Until recently, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) were not recommended by EULAR to treat enthesitis [6]. However, the SEAM-PsA trial (NCT02376790) changed this view. This phase 3, randomised, double-blind study included 851 participants with PsA and aimed to examine the efficacy of methotrexate monotherapy compared with etanercept monotherapy and the value of combining methotrexate and etanercept [7]. In this trial, etanercept monotherapy and combination therapy showed greater efficacy than methotrexate monotherapy. However, even with methotrexate monotherapy, 50.7% of participants achieved an ACR20 response with an enthesitis resolution in 43.5% of cases [8]. Furthermore, “Methotrexate is actually linked to non-evolution to PsA when used in subjects with psoriasis,” added Prof. McGonagle. This was recently shown in a retrospective study, where the incidence of PsA in patients with psoriasis was assessed according to different treatments for their skin: topics/no treatment, csDMARDs, and biological DMARDs (bDMARDs). During follow-up, the incidence of PsA was lowest in patients treated with biological agents (1.9%), followed by those treated with csDMARDs (6%), and as high as 16.6% in patients treated with topical therapy only [8]. Therefore, in the updated GRAPPA 2021 recommendations on PsA, methotrexate can be used to treat enthesitis [9]. Other csDMARDS including sulphasalazine need revisiting and overall more work is needed in this space to determine its role in a predominant enthesitis pattern of PsA or SpA.
Efficacy of IL-17 and IL-23 blockers
The anti-TNF agents have been used for over a decade to treat enthesitis and the question that has recently been addressed is whether the IL-23/17 axis cytokine blockade might be better for enthesitis. IL-17 inhibitors can be used to treat enthesitis. In the open-label, head-to-head trial SPIRIT-H2H (NCT03151551), ixekizumab was superior to adalimumab regarding the resolution of enthesitis at week 24, and numerically superior at week 52, even though this was not the primary outcome [10,11]. Moreover, IL-23 blockers have shown to be efficacious in treating enthesitis [12]. However, in the double-blind, phase 3, EXCEED trial (NCT02745080), resolution rates of enthesitis were similar when secukinumab was compared with adalimumab [13]. In a network meta-analysis of phase 3 studies on the PsA treatment, the efficacy and safety of different DMARDs were compared, with a special focus on bDMARDs [14]. According to this analysis, guselkumab, IL-17 inhibitors (secukinumab, ixekizumab, brodalumab), and adalimumab were similarly efficacious in the resolution of enthesitis (see Figure). “Although this is not clear yet, IL-17 and IL-23 blockers might be more efficacious than TNF-blockers,” Prof. McGonagle said. Head-to-head studies comparing different mechanisms of action with enthesitis resolution as the primary outcome will be needed to evaluate this.
Figure: Network meta-analysis of enthesitis resolution in RCTs of PsA where ACR20 was the primary outcome [14]
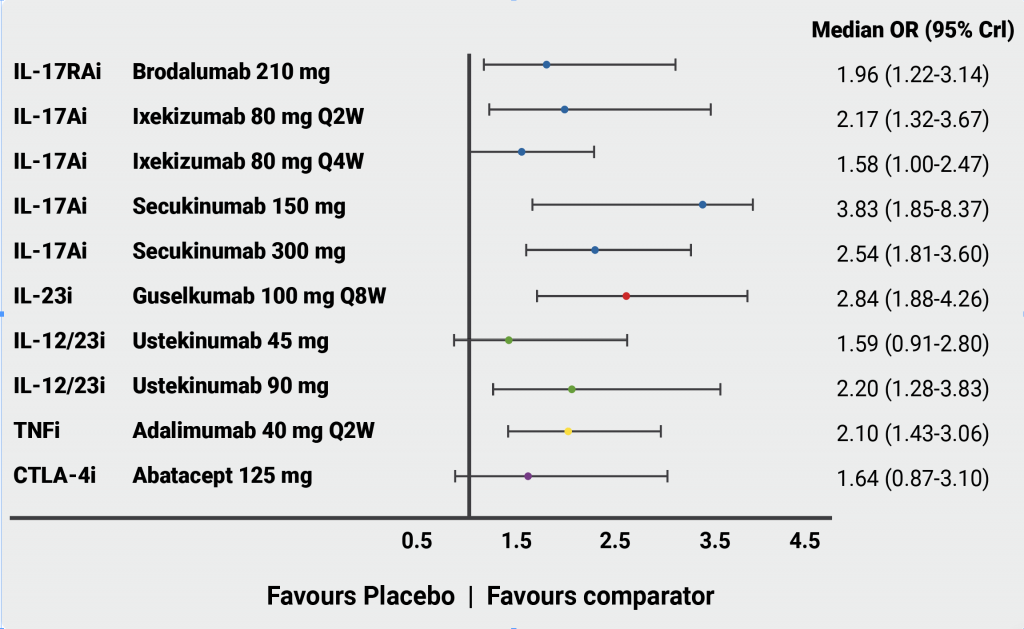
Q2W, every 2 weeks; Q4W, every 4 weeks; Q8W, every 8 weeks; Crl, credible interval.
Reprinted and adjusted from McInnes IB, et al. RMD Open. 2022:8:e002074. doi: 10.1136/rmdopen-2021-002074 under the terms of the Creative Commons Attribution 4.0 license.
Microinflammation and healing are common in enthesitis. “That is why alternative medicine and placebo do so well,” Prof. McGonagle said. This reflects the normal cycle of microdamage and subsequent tissue repair responses. For the physician asking the question of what is the best agent to treat a predominant enthesitis pathology, Prof. McGonagle concluded: “There is no clear evidence that one class of drug is superior to another in the therapy of enthesitis, but further research is needed.”
- McGonagle D. Enthesitis: how do we treat it in 2022? EULAR 2022 Congress, 1–4 June, Copenhagen, Denmark.
- Kavanaugh A, et al. Rheumatol Ther. 2016;3:91–102.
- McGonagle D, et al. Lancet. 1998;352:1137–40.
- Bridgewood C, et al. Immunol Rev. 2020;294:27–47.
- Healy PJ, Helliwell PS. Arthritis Rheum. 2008;59:686–91.
- Gossec L, et al. Ann Rheum Dis. 2020;79:700–12.
- Mease PJ, et al. Arthr Rheumatol. 2019;71:1112–24.
- Felquer MLA, et al. Ann Rheum Dis. 2022;81:74–9.
- Coates LC, et al. Ann Rheum Dis. 2021;80:139–140.
- Mease PJ, et al. Ann Rheum Dis. 2020;79:123–31.
- Smolen JS, et al. Ann Rheum Dis. 2020;79:1310–9.
- Östör AR, et al. Ann Rheum Dis. 2022;81:351–8.
- McInnes IB, et al. Lancet. 2020;395:1496-1505.
- McInnes IB, et al. RMD Open. 2022;8:e002074.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Baseline cardiovascular risk linked to higher rates of MACE in PsA and PsO patients receiving tofacitinib Next Article
Body mass index increase associated with structural changes in knee OA »
« Baseline cardiovascular risk linked to higher rates of MACE in PsA and PsO patients receiving tofacitinib Next Article
Body mass index increase associated with structural changes in knee OA »
Table of Contents: EULAR 2022
Featured articles
Late-Breaking Oral Abstracts
TYK2 inhibition: the future of treating lupus erythematosus?
Psoriatic arthritis: significant improvement with bimekizumab
Baricitinib could open the door to oral treatment for juvenile idiopathic arthritis
Sarilumab for polymyalgia rheumatica led to sustained remission and fewer flares
Spotlight on Rheumatoid Arthritis
Comorbid depression comes with a profoundly higher mortality risk in RA
Preventive treatment with methotrexate benefits pre-RA patients with arthralgia
Risk factors for dementia in RA patients discovered
VTE in global registry data more common in JAK inhibitor-treated RA patients
Spondyloarthropathies – Novel Developments
How to treat enthesitis in 2022
Baseline cardiovascular risk linked to higher rates of MACE in PsA and PsO patients receiving tofacitinib
Treat-to-target dose reduction effective in spondyloarthritis
A novel oral treatment possibility for non-radiographic axSpA on the horizon
Many RA and PsA patients have problems with their sex life
What Is Hot in Osteoarthritis?
New treatments in osteoarthritis
OA associated with alcohol and drug abuse
Body mass index increase associated with structural changes in knee OA
What Is New in Lupus and Scleroderma
Inhibition of Bruton’s tyrosine kinase: a new way of approaching SLE?
Pregnancies in SLE: many complications for mothers and their unborn children
Lupus nephritis: Efficient treatment may reduce the risk of kidney disease advancement
Antifibrotic therapy with nintedanib is beneficial for patients with negative prognostic factors
Best of the Posters
Alarmingly low activity in patients with non-inflammatory and inflammatory rheumatic disease
High prevalence of fibromyalgia in patients with inflammatory bowel disease
Related Articles
November 12, 2021
Anti-TNF therapy tied to better outcomes in IBD patients with COVID-19
October 23, 2019
IBD prevalence 3 times higher than estimated and expected to rise
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

