https://doi.org/10.55788/ebf2b4a6
PsA and PsO are linked to an increased burden of CV and metabolic comorbidities, and an association between CV disease and future development of malignant tumours has been described [1-4]. Recent concerns about the cardiovascular safety associated with JAK inhibitors in patients with rheumatoid arthritis have arisen. To explore this issue further, Prof. Lars Erik Kristensen (Copenhagen University Hospital, Denmark) and his fellow researchers performed a post-hoc analysis regarding patients with PsA and PsO treated with tofacitinib [1]. The analysed data comprised 3 PsA and 7 PsO trials of phase 2 and 3, as well as open-label extensions. Outcomes were defined as incidence rates for MACE and malignancies, the latter excluding non-melanoma skin cancer. Risk stratifications were performed according to the history of coronary artery disease, the baseline 10-year risk of atherosclerotic CV disease, and baseline metabolic syndrome. The follow-up lasted until the occurrence of the first event or 28 days after the last administration of tofacitinib.
Overall, 783 PsA patients with 2,038 patient-years of tofacitinib exposure and 3,663 PsO patients with a total exposure of 8,950 patient-years were included. Median exposure time was 3.0 and 2.4 years in patients with PsA and PsO, respectively. History of coronary artery disease was present in 5% and 2.5%, as well as baseline metabolic syndrome in 40.9% and 32.7%, respectively. The patients without coronary artery disease were stratified in correspondence to their baseline atherosclerotic CV disease risk, according to the Pooled Cohort Equations calculator, and more than 20% turned out to be in the intermediate or high-risk category.
The incidence rates for MACE in PsA patients ranged from 0.1 (95% CI 0.0–0.4) to 1.3 (95% CI 0.0–7.0), with increasing values associated with increasing baseline atherosclerotic CV risk. The presence of baseline metabolic syndrome was linked to an incidence rate of 0.6 (95% CI 0.2–1.4). “For PsO patients, we saw the same pattern, also an increasing incident rate with increasing background risk,” Prof. Kristensen explained. He put these results into perspective by pointing out some imprecision due to rather low amounts of events leading to wide confidence intervals of the incidence rates, both for MACE and malignancies. Indeed, in terms of malignancies, the highest incidence rates were observed in patients with intermediate (PsA: 2.5 [95% CI 1.1–4.9] and PsO: 1.3 [95% CI 0.8–1.9]) and high (PsA: 1.3 [95% CI 0.0–7.0] and PsO: 3.6 [95% CI 2.0–5.9]) atherosclerotic CV risk (see Figure). The numerically higher rates for PsO related to greater drug exposure in terms of patient-years.
Figure: Incidence rates of malignancies in patients with PsA treated with tofacitinib [1]
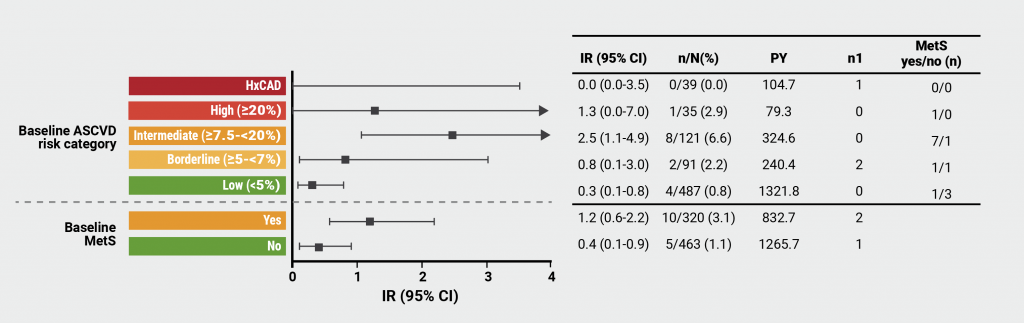
ASCVD, atherosclerotic cardiovascular disease; MetS, metabolic syndrome; HxCAD, history of coronary artery disease; IR, incidence rate; CI, confidence interval; n, patients with malignancies; N, total number of patients; n1, number of patients with first event outside the risk period; PY, patient-years.
“This further stresses that we should look beyond the disease targets, the skin and the joints. We should also care about comorbidities, especially cardiovascular ones, and treat those. I think we, as rheumatologists should be in the centre and be responsible, we might delegate to primary care and so on, but it is an important outcome,” Prof. Kristensen stressed in his conclusion.
- Kristensen LE, et al. Association between baseline cardiovascular risk and incidence rates of major adverse cardiovascular events and malignancies in patients with psoriatic arthritis and psoriasis receiving tofacitinib. OP0027, EULAR 2022 Congress, 1–4 June, Copenhagen, Denmark.
- Karmacharya P, et al. Ther Adv Musculoskelet Dis. 2021;13:1759720X21998279.
- Garshick MS, et al. J Am Coll Cardiol. 2021;77:1670–1680.
- Lau ES, et al. JACC CardioOncol. 2021;3:48–58.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Treat-to-target dose reduction effective in spondyloarthritis Next Article
How to treat enthesitis in 2022 »
« Treat-to-target dose reduction effective in spondyloarthritis Next Article
How to treat enthesitis in 2022 »
Table of Contents: EULAR 2022
Featured articles
Late-Breaking Oral Abstracts
TYK2 inhibition: the future of treating lupus erythematosus?
Psoriatic arthritis: significant improvement with bimekizumab
Baricitinib could open the door to oral treatment for juvenile idiopathic arthritis
Sarilumab for polymyalgia rheumatica led to sustained remission and fewer flares
Spotlight on Rheumatoid Arthritis
Comorbid depression comes with a profoundly higher mortality risk in RA
Preventive treatment with methotrexate benefits pre-RA patients with arthralgia
Risk factors for dementia in RA patients discovered
VTE in global registry data more common in JAK inhibitor-treated RA patients
Spondyloarthropathies – Novel Developments
How to treat enthesitis in 2022
Baseline cardiovascular risk linked to higher rates of MACE in PsA and PsO patients receiving tofacitinib
Treat-to-target dose reduction effective in spondyloarthritis
A novel oral treatment possibility for non-radiographic axSpA on the horizon
Many RA and PsA patients have problems with their sex life
What Is Hot in Osteoarthritis?
New treatments in osteoarthritis
OA associated with alcohol and drug abuse
Body mass index increase associated with structural changes in knee OA
What Is New in Lupus and Scleroderma
Inhibition of Bruton’s tyrosine kinase: a new way of approaching SLE?
Pregnancies in SLE: many complications for mothers and their unborn children
Lupus nephritis: Efficient treatment may reduce the risk of kidney disease advancement
Antifibrotic therapy with nintedanib is beneficial for patients with negative prognostic factors
Best of the Posters
Alarmingly low activity in patients with non-inflammatory and inflammatory rheumatic disease
High prevalence of fibromyalgia in patients with inflammatory bowel disease
Related Articles
November 6, 2024
Deucravacitinib treatment in lichen planopilaris

April 18, 2024
How effective is dose escalation of biologicals in IBD?
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

