“Locally advanced rectal cancer is treated with a combination of chemotherapy, radiation, and optional surgery, with all its costs and life-altering consequences,” said Dr Andrea Cercek (Memorial Sloan Kettering Cancer Center, NY, USA) [1]. Patients with dMMR rectal cancer are relatively resistant to chemotherapy but are responsive to checkpoint inhibitors [2,3]. The current phase 2 study aimed to assess whether neoadjuvant PD-1 inhibition may be able to replace chemotherapy, chemo- and radiation therapy, or chemotherapy, radiation therapy, and surgery in patients with dMMR rectal cancer.
For this purpose, 30 patients received dostarlimab (500 mg, intravenous, every 3 weeks) for 6 months followed by radiologic and endoscopic evaluation. If a clinical complete response was achieved, a wait-and-see approach was installed with 4-monthly follow-up visitations. If residual disease was detected, patients received chemoradiation. After completion of this therapy, another evaluation would decide if surgery was needed. The primary objectives were the overall response rate of PD-1 blockade with or without chemoradiation and the pathologic or clinical complete response rate at 12 months after PD-1 therapy with or without chemoradiation.
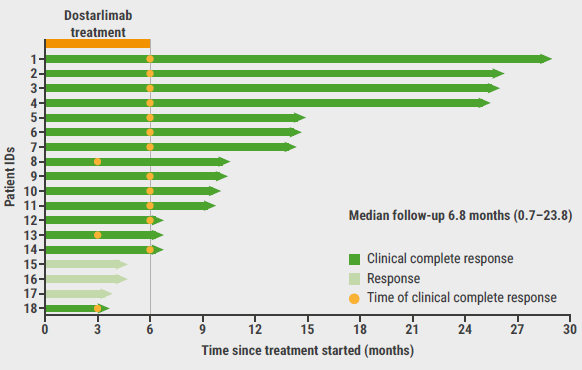
Spectacularly, all 14 patients that have been analysed to date reached a clinical complete response on dostarlimab therapy alone. Moreover, the responses were ongoing in all assessed patients after a median follow-up of 6.8 months (see Figure). Furthermore, no grade 3 or 4 adverse events were reported in these patients.
Figure: Duration of clinical complete response on dostarlimab treatment [3]
According to Dr Cercek, PD-1 inhibition may eliminate the need for chemotherapy and radiation in patients with early-stage dMMR rectal cancer and may rapidly translate to areas without access to modern chemotherapy, radiation, and surgery. It will spare patients from toxicity and late effects of chemo- and radiation therapy and surgery.
“Based on the data of this study, off-protocol use of neoadjuvant immunotherapy in this population is likely to occur,” expected Dr Kimmie Ng (Dana-Farber Cancer Institute, MA, USA), who commented on this late-breaking abstract. Dr Ng commented that the results are spectacular, but that larger sample sizes, longer follow-up time, and results from other endpoints are needed before standard-of-care may be changed in this population.
- Cercek A, et al. Single agent PD-1 blockade as curative-intent treatment in mismatch repair deficient locally advanced rectal cancer. LBA5, ASCO 2022 Annual Meeting, 3–7 June, Chicago, IL, USA.
- Cercek A, et al. Clin Can Res. 2020;26(13):3271‒3279.
- Andre T, et al. NEJM. 2020;383;2207–2218.
Copyright ©2022 Medicom Medical Publishers
Posted on