https://doi.org/10.55788/f64b73ba
“The HER2-status of patients with breast cancer is defined by immunohistochemistry (IHC) scores, with HER2-low being defined as having an IHC score of 1+ or 2+ with in situ hybridisation (ISH)-negative status,” explained Prof. Shanu Modi (Memorial Sloan Kettering Cancer Center, NY, USA). The options for these patients, especially in later lines of therapy, are limited [1]. The DESTINY-Breast04 trial (NCT03734029) randomised patients with HER2-low unresectable or metastatic breast cancer to T-DXd (n=373) or chemotherapy by physician’s choice (n=184) [2]. Patients had received 1 or 2 prior lines of chemotherapy and were refractory to endocrine therapy if they were HR-positive. PFS by independent central review was the primary endpoint of this study. Of note, approximately 90% of the patients were HR-positive, whereas 10% were HR-negative.
The median PFS of patients on T-DXd was superior to that of patients on chemotherapy (9.9 vs 5.1 months; HR 0.50; P<0.0001) and the same was true for the HR-positive subset of patients (10.1 vs 5.4 months; HR 0.51; P<0.0001; see Figure). Moreover, the median OS favoured T-DXd over chemotherapy in the general population (23.4 vs 16.8 months; HR 0.64; P=0.0010) and in the HR-positive subset of patients (23.9 vs 17.5 months; HR 0.64; P=0.0028). An exploratory analysis displayed that HR-negative patients are likely to benefit from T-DXd as well in terms of PFS (8.5 vs 2.9 months; HR 0.46) and OS (18.2 vs 8.3 months; HR 0.48). The results were consistent across subgroups.
Figure: Progression-free survival of HR-positive patients versus the full analysis set [2]
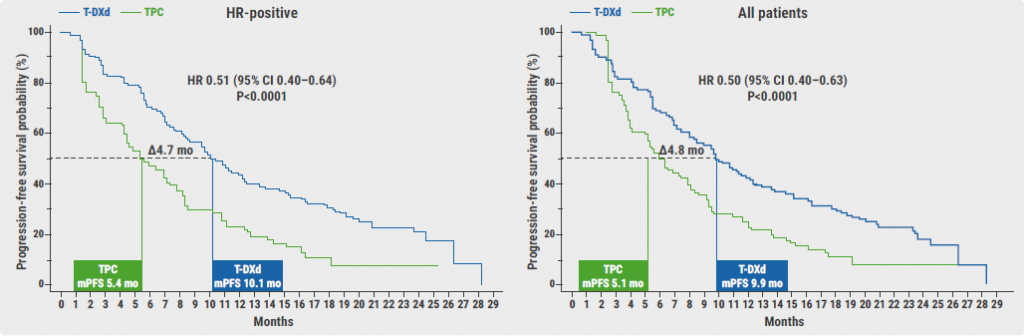
T-DXd, Trastuzumab deruxtecan; TPC, chemotherapy by physician’s choice; mPFS, median progression-free survival; mo, months.
The safety analysis did not reveal new safety issues. Neutropenia was more frequently observed in the chemotherapy arm, whereas nausea was more often reported in the T-DXd arm. Prof. Modi added that the cases of nausea were mostly grade 1 or 2 events, which should be manageable in practice. In total, 16% of the patients in the T-DXd arm experienced treatment-emergent adverse events that led to dose discontinuation, compared with 8% in the chemotherapy arm. ILD/pneumonitis occurred in 12.1% of the patients on T-DXd: 3.5% grade 1, 6.5% grade 2, 1.3% grade 3, and 0.8% grade 5 events. Decreased left ventricular ejection fraction occurred in 4.3% of the patients on T-DXd: 0.3% grade 1, 3.8% grade 2, and 0.3% grade 3.
“T-DXd is the first HER2-targeted therapy to demonstrate improved efficacy in HER2-low metastatic breast cancer, establishing a new standard-of-care for this population, which covers approximately 50% of the total metastatic breast cancer population,” concluded Dr Modi.
- Tarantino P, et al. J Clin Oncol. 2020;38(17):1951–1962.
- Modi S, et al. Trastuzumab deruxtecan (T-DXd) versus treatment of physician’s choice (TPC) in patients (pts) with HER2-low unresectable and/or metastatic breast cancer (mBC): Results of DESTINY-Breast04, a randomized, phase 3 study. LBA3, ASCO 2022 Annual Meeting, 3–7 June, Chicago, IL, USA.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« SET2,3 to inform on chemotherapy decisions in ER-positive breast cancer Next Article
Shaky OS results of palbociclib in ER-positive/HER2-negative breast cancer »
« SET2,3 to inform on chemotherapy decisions in ER-positive breast cancer Next Article
Shaky OS results of palbociclib in ER-positive/HER2-negative breast cancer »
Table of Contents: ASCO 2022
Featured articles
Breast Cancer
Sacituzumab govitecan meets primary endpoint
Shaky OS results of palbociclib in ER-positive/HER2-negative breast cancer
Practice-changing results of T-DXd in HER2-low breast cancer
SET2,3 to inform on chemotherapy decisions in ER-positive breast cancer
Metastasis-directed therapy fails in oligometastatic breast cancer
Analysis by residual cancer burden further clarifies effect of pembrolizumab
Contribution of metastatic therapies on mortality reduction in breast cancer
Radiotherapy may be omitted in breast cancer patients
Promising data for ribociclib after progression on ET plus CDK4/6 inhibitors in HR-positive/HER2-negative metastatic breast cancer
7-gene biosignature: Benefits of endocrine therapy and radiotherapy in breast cancer risk groups
Lung Cancer
Additional tiragolumab does not help patients with untreated small cell lung cancer
Success for serplulimab plus chemotherapy in small cell lung cancer
Adagrasib safe and clinically active in non-small cell lung cancer
Long-term benefits of combined immunotherapy over chemotherapy in non-small cell lung cancer
Effect of KRAS mutations and PD-L1 expression on therapy response in non-small cell lung cancer
Melanoma
First results on distant metastasis-free survival in stage II melanoma
Higher response rates for concurrent triple therapy versus sequential therapy in melanoma
Genitourinary Cancers
Exploratory treatment options fail in ccRCC
Adjuvant everolimus did not benefit high-risk renal cell carcinoma
Cabozantinib fails as first-line maintenance therapy in urothelial cancer
177Lu-PSMA-617 is a valid treatment option for PSMA-positive mCRPC
Enzalutamide performs well in metastatic hormone-sensitive prostate cancer
Haematologic Malignancies
Autologous stem cell transplantation plus RVd improves PFS in multiple myeloma
Novel first-line treatment option for mantle cell lymphoma
Promising results for novel CAR-T therapy in relapsed/refractory multiple myeloma
Gastrointestinal Cancers
Panitumumab beats bevacizumab in RAS wildtype left-sided metastatic colorectal cancer
Spectacular results for dostarlimab in mismatch repair deficient rectal cancer
Triplet chemotherapy beats doublet chemotherapy in colorectal cancer liver metastases
To resect or not to resect primary tumours in stage IV colon cancer?
Novel treatment option for KRAS wildtype pancreatic cancer
Gynaecological Cancers
Primary results of rucaparib in ovarian cancer
Trabectedin not superior to chemotherapy in recurrent epithelial ovarian cancer
Encouraging results of relacorilant in ovarian cancer
Miscellaneous Topics
Bacterial decolonisation effective against radiation dermatitis
New standard-of-care for cisplatin-ineligible locally advanced head and neck squamous cell carcinoma
Ifosfamide is likely to be the go-to therapy in recurrent Ewing sarcoma
Dabrafenib plus trametinib candidates for standard-of-care in BRAF V600-mutated paediatric low-grade glioma
Related Articles

November 26, 2019
Preoperative chemotherapy for colon cancer
March 21, 2022
DESTINY-CRC01: Maintained efficacy of T-DXd in mCRC
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

