PDP is progressive, debilitating, and common, with a prevalence of around 60% as Parkinson’s disease progresses. Most treatment options are limited due to lack of efficacy, safety concerns, and/or exacerbation of motor symptoms. SEP-363856 is a full agonist of the TAAR1 and 5HT1A receptors. It is a potent regulator of dopamine neurotransmission and represents a novel way to prevent hyperactivity of the dopaminergic system. In a previous study, SEP-363856 demonstrated antipsychotic-like activity [1]. A proof-of-principle study evaluated safety and efficacy of SEP-363856 in PDP patients [2]. The results were shared by Dr Stuart Isaacson (Parkinson's Disease and Movement Disorders Center of Boca Raton, FL, USA).
PDP patients requiring treatment were randomised to SEP-363856 (25, 50, or 75 mg/day) or placebo. Primary endpoint was change in Scale for Assessment of Positive Symptoms–Parkinson’s Disease (SAPS-PD) after 6 weeks. The intention-to-treat population included 38 patients (SEP-363856, n=24; placebo, n=14). Mean time since onset of Parkinson’s disease and PDP were 9.0 and 2.4 years, respectively. Mean baseline SAPS-PD total score was 13.6; mean Mini-Mental State Examination (MMSE) score was 25.0.
Improvements in SAPS-PD total scores for SEP-363856 compared with placebo occurred as early as week 1; least-squares mean change from baseline at week 6 was -2.5 versus -1.4 (P=0.681; see Figure). A ≥100% improvement in SAPS-PD total score was seen in 25% versus 0% of patients. Regarding SAPS-PD subscores, there was a greater effect of SEP-363856 on hallucinations (-3.6 vs -1.9; P=0.339). Dr Isaacson also noted that SEP-363856 was relatively more effective in patients with cognitive impairment. SAPS-PD total score in patients with baseline MMSE score ≤24 was -5.2 versus -2.1 (P=0.460). No change at 6 weeks in motor parkinsonism was noted.
Figure: SAPS-PD total score over 6 weeks [2]
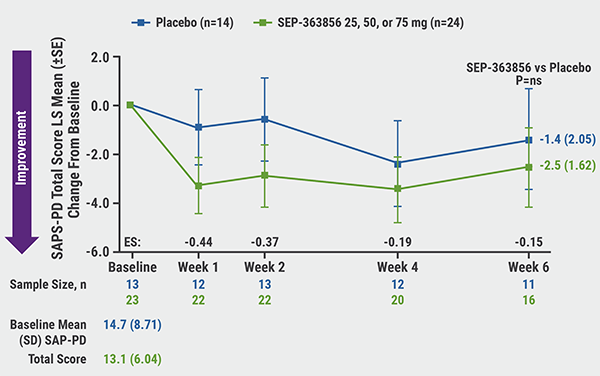
SAPS-PD, Scale for Assessment of Positive Symptoms–Parkinson’s Disease; SE, standard error; LS, least squares; ES, effect size.
Adverse events occurring in ≥15% with SEP-363856 versus placebo included hallucinations (24% vs 14%), confused state (20% vs 14%), dizziness (16% vs 7%), and falls (12% vs 21%). CNS adverse events seemed dose-related. Adequately powered studies are needed to confirm these findings.
- Koblan KS, et al. N Engl J Med. 2020;382(16):1497-1506.
- Isaacson S, et al. Efficacy and Safety of SEP-363856, a Non–D2-Receptor Binding Drug With Antipsychotic Activity, in Patients With Parkinson’s Disease Psychosis. S3.005, AAN 2021 Virtual Congress, 17-22 April.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Troriluzole for spinocerebellar ataxia Next Article
Stable long-term effects of deep brain stimulation in Parkinson’s disease »
« Troriluzole for spinocerebellar ataxia Next Article
Stable long-term effects of deep brain stimulation in Parkinson’s disease »
Table of Contents: AAN 2021
Featured articles
Letter from the Editor
Interview with AAN President Dr James C. Stevens
COVID-19 and Neurology
The neurological impact of COVID-19
Chemosensory dysfunction often persistent after COVID-19
Pandemic results in decreased global stroke care
Stroke uncommon in critically ill COVID-19 patients
Cognitive Impairment and Dementias
Obstructive sleep apnoea associated with lower cognition
NfL is a better marker for neurodegeneration than T-tau
Monoclonal antibody rapidly reduces brain amyloid
Epilepsy
Extraordinary transformation of epilepsy care in Ontario
No neurodevelopmental effects of foetal antiseizure medication
Migraine and Other Headaches
Long-term safety of atogepant as migraine prophylaxis
Multiple Sclerosis
Dysmetabolism may drive MS progression
Predicting long-term prognosis in paediatric MS patients
Neuromuscular Disorders
Functional and survival benefits of AMX0035 in ALS
Parkinson’s Disease and Other Movement Disorders
Autoimmune mechanisms implicated in Parkinson’s disease
Novel non–D2-receptor-binding treatment for Parkinson’s disease psychosis
Troriluzole for spinocerebellar ataxia
Stroke
Can linoleic acid help prevent stroke?
No association between SSRIs and risk of ICH
Other Topics
Vutrisiran for hATTR amyloidosis with polyneuropathy
10 kHz spinal cord stimulation for painful diabetic neuropathy
Related Articles
May 21, 2019
Penile prosthesis implantation
February 18, 2021
“Strongly consider an SGLT2-inhibitor in most T2DM patients”
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

