A total of 844 MS patients were enrolled in the study; 565 had suspected COVID-19 and 279 confirmed COVID-19 [1]. Groups were classified based on COVID-19 severity as 1) mild disease not requiring hospitalisation; 2) pneumonia or hospitalisation; 3) intensive care unit (ICU) admission or death. Mean age was 45 years, 593 (70.3%) were women. The median expanded disability status scale (EDSS) score was 2; 16% had progressive MS.
Of the included patients, 38 (4.5%) were admitted to an ICU, 99 (11.7%) had pneumonia, and 96 (11.4%) were hospitalised. The number of fatalities was 13 (1.54%), 11 of which had progressive MS; 8 were not on any therapy.
The use of anti-CD20 therapy (ocrelizumab or rituximab) was significantly associated with increased risk of severe COVID-19 (OR 2.37; 95% CI 1.18–4.74; P=0.015). Recent use (<1 month) of methylprednisolone was also associated with a worse outcome (OR 5.24; 95% CI 2.20–12.53; P=0.001). In contrast, a protective effect against severe COVID-19 was observed for interferon (OR 0.36) and teriflunomide (OR 0.49, trending). Additional analysis revealed that duration of anti-CD20 treatment may be associated with severe infection (≤6 months: OR 1.56; >2 years: OR 2.75). Other factors that significantly impacted infection severity were age, sex, EDSS scores, presence of comorbidities, and high-dose steroid use one month before symptoms (see Table).
Table: Risk factors for severe COVID-19 in 1,584 confirmed cases: multivariate analysis [1]
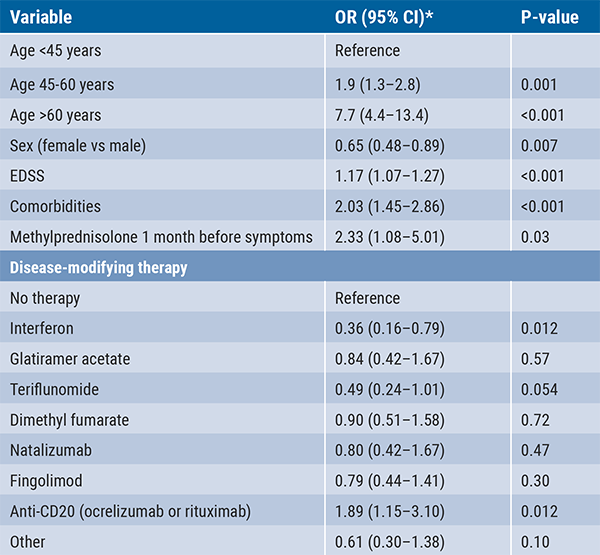
*Ordinal logistic regression applies: the outcome is a severity scale that is “ordered”: Level 0 = mild disease, no pneumonia, not requiring hospitalisation (n=1,363); Level 1 = pneumonia or hospitalisation (n=184); Level 2 = ICU or death (n=36). The OR represents the risk increase passing from one classification to the next.
- Sormani MP, et al. Different disease modifying therapies can increase or decrease COVID-19 severity in Multiple Sclerosis. S28.002, AAN 2021 Virtual Congress, 17-22 April.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Soticlestat in children with Dravet and Lennox-Gastaut Next Article
Durable seizure frequency reduction with cenobamate »
« Soticlestat in children with Dravet and Lennox-Gastaut Next Article
Durable seizure frequency reduction with cenobamate »
Related Articles
November 25, 2020
Outcomes in patients on alemtuzumab with thyroid adverse events
September 10, 2020
Eculizumab in NMOSD: the PREVENT study

December 19, 2022
Cerebrospinal fluid kappa-free light chains for MS diagnosis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

