Zavegepant is the only intranasal CGRP receptor antagonist in late-stage development for the acute treatment of migraine. In the presented trial (NCT03872453), subjects with a single attack of migraine with moderate-to-severe pain intensity were treated with intranasal zavegepant 5, 10, 20 mg, or placebo [1]. A total of 1,673 adults with migraine were randomised, 1,581 of whom were included in the intention-to-treat population. Median age was 40 years, 85.5% were female, and 14% took prophylactic migraine medication.
The co-primary endpoints of freedom from pain and freedom from MBS were significantly more often reached in the zavegepant 10 mg and 20 mg groups than in the placebo group (see Figure). The onset of pain relief occurred as early as 15 minutes post-dose. There was no significant difference in the secondary endpoint of pain relief at 2 hours post-dose (P=0.0167) for any zavegepant dose group.
Figure: Intranasal zavegepant is superior to placebo on co-primary endpoints of a phase 2/3 trial [1]
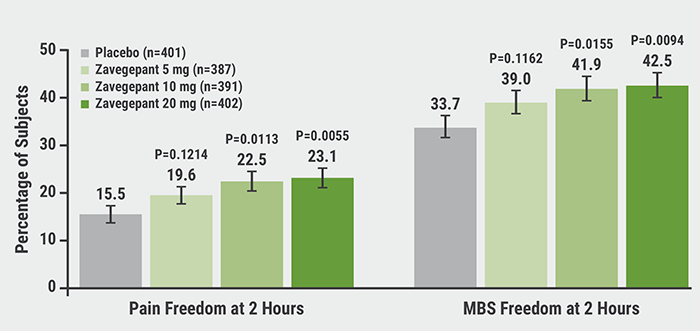
MBS, most bothersome symptom (photophobia, phonophobia, or nausea).
The most common (>5%) adverse events were dysgeusia (13.5–16.1% with zavegepant vs 3.5% with placebo) and nasal discomfort (1.3–5.2% with zavegepant vs 0.2% with placebo). The majority of adverse events were mild or moderate and not related to the study drug. There was no signal of hepatoxicity.
- Croop R, et al. Intranasal Zavegepant is Effective and Well Tolerated for the Acute Treatment of Migraine: A Phase 2/3 Dose-Ranging Clinical trial. S5.003, AAN 2021 Virtual Congress, 17-22 April.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Chemosensory dysfunction often persistent after COVID-19 Next Article
Natalizumab versus fingolimod in subgroups of MS patients »
« Chemosensory dysfunction often persistent after COVID-19 Next Article
Natalizumab versus fingolimod in subgroups of MS patients »
Related Articles
August 18, 2021
Rhythmicity in primary headache disorders
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

