LXB contains calcium, magnesium, potassium, and sodium oxybate, representing a novel oxybate treatment with 92% less sodium than SXB. It was tested in patients with idiopathic hypersomnia, a condition for which currently no approved treatment exists [1]. All participants started with 16 weeks of treatment with LXB, during which period the dose was titrated, optimised and, during the last 2 weeks, stabilised. They were then randomised to placebo or to continue LXB for a 2-week withdrawal period. The primary efficacy endpoint was change in Epworth Sleepiness Scale (ESS) score. The safety population included 154 participants, who had a mean ESS of 16; mean dose was 6.0 g/night.
At the end of the double-blind withdrawal period, ESS scores were significantly worse in the placebo group compared with the LXB group, with a mean difference in change of -6.51 (95% CI -7.99 to -5.03; P<0.0001). Patient Global Impression of Change (PGIc) was also significantly worse (21.4% for LXB vs 88.1% for placebo; P<0.0001), as was Idiopathic Hypersomnia Severity Scale (IHSS) score, with an estimated median difference of -12.00 (95% CI -15.0 to -8.0; P<0.0001). Overall incidence of treatment-emergent adverse events was 80%. Treatment-emergent adverse events which occurred in at least 5% of participants were nausea (21.4%), headache (16.2%), dizziness (11.7%), anxiety (10.4%), and vomiting (10.4%). Serious treatment-emergent adverse events occurred in 4 participants, but none were deemed related to study drug.
Results of LXB in patients with narcolepsy with cataplexy were also presented [2]. All participants received LXB during a 12-week optimised treatment and titration period, followed by a 2-week stable-dose period. They were then randomised to placebo or continued LXB treatment during a 2-week withdrawal period. Health-related quality of life worsened in those randomised to placebo but remained stable in participants who continued LXB treatment. The overall safety profile of LXB was similar to SXB.
- Dauvilliers Y, et al. Efficacy and Safety of Lower-Sodium Oxybate in a Phase 3, Placebo-Controlled, Double-Blind, Randomized Withdrawal Study in Adult Participants With Idiopathic Hypersomnia. AAN 2021 Virtual Congress, 17-22 April.
- Foldvary-Schaefer N, et al. Quality of Life in a Phase 3, Placebo-Controlled, Double-Blind, Randomized Withdrawal Study of Lower-Sodium Oxybate in Adults With Narcolepsy With Cataplexy. S9.002, AAN 2021 Virtual Congress, 17-22 April.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Can linoleic acid help prevent stroke? Next Article
Troriluzole for spinocerebellar ataxia »
« Can linoleic acid help prevent stroke? Next Article
Troriluzole for spinocerebellar ataxia »
Table of Contents: AAN 2021
Featured articles
Letter from the Editor
Interview with AAN President Dr James C. Stevens
COVID-19 and Neurology
The neurological impact of COVID-19
Chemosensory dysfunction often persistent after COVID-19
Pandemic results in decreased global stroke care
Stroke uncommon in critically ill COVID-19 patients
Cognitive Impairment and Dementias
Obstructive sleep apnoea associated with lower cognition
NfL is a better marker for neurodegeneration than T-tau
Monoclonal antibody rapidly reduces brain amyloid
Epilepsy
Extraordinary transformation of epilepsy care in Ontario
No neurodevelopmental effects of foetal antiseizure medication
Migraine and Other Headaches
Long-term safety of atogepant as migraine prophylaxis
Multiple Sclerosis
Dysmetabolism may drive MS progression
Predicting long-term prognosis in paediatric MS patients
Neuromuscular Disorders
Functional and survival benefits of AMX0035 in ALS
Parkinson’s Disease and Other Movement Disorders
Autoimmune mechanisms implicated in Parkinson’s disease
Novel non–D2-receptor-binding treatment for Parkinson’s disease psychosis
Troriluzole for spinocerebellar ataxia
Stroke
Can linoleic acid help prevent stroke?
No association between SSRIs and risk of ICH
Other Topics
Vutrisiran for hATTR amyloidosis with polyneuropathy
10 kHz spinal cord stimulation for painful diabetic neuropathy
Related Articles
January 14, 2022
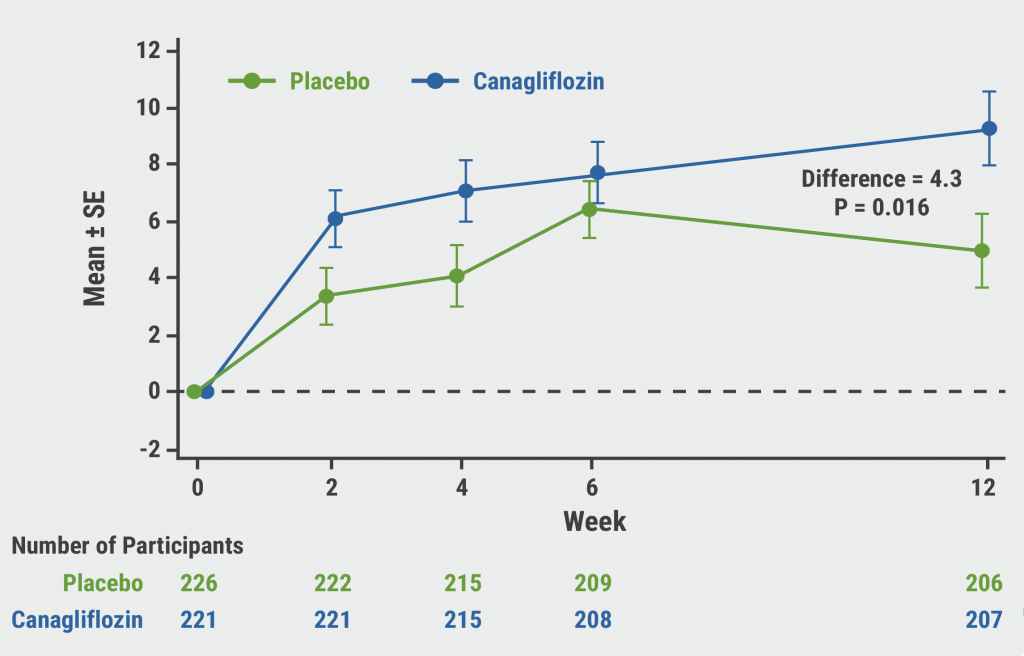
CHIEF-HF: Canagliflozin improves health status in heart failure
October 30, 2023
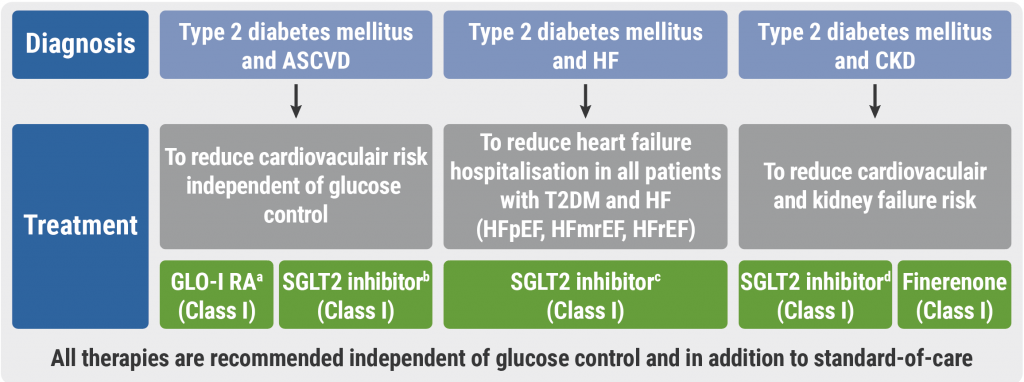
Cardiovascular disease and diabetes: new guidelines
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

