
Medicom Medical Publishers spoke with lead investigator Dr Eliza Hawkes (Olivia Newton-John Cancer Wellness & Research Centre, Australia) about how WAMM explores a novel approach to treating MCL, namely through the use of acalabrutinib, a highly selective Bruton’s tyrosine kinase (BTK) inhibitor, in a unique and innovative ‘sandwich’ design [1]. BTK inhibitors have improved first-line therapy efficacy in both elderly and young, fit, MCL; the latter with intermittent dosing during R-CHOP in an alternating R-CHOP/cytarabine-based regimen [2,3].
The WAMM study enrolled patients between the ages of 18 and 70 years with previously-untreated, histologically-proven, CD20-positive, stage II–IV MCL (all subtypes). Eligible patients were required to have an ECOG performance status of 0–1 and no comorbidities that would preclude stem cell transplantation (SCT). The primary goal of the WAMM study is to evaluate the safety and efficacy of the acalabrutinib sandwich therapy. Specifically, the study assesses the rate of complete metabolic response (CMR) after induction therapy with acalabrutinib and rituximab, followed by R-DHAOx chemoimmunotherapy. Additionally, the study evaluates the overall response rate, overall toxicity, overall and progression-free survival, minimal residual disease (MRD) negativity rates, and standardised quality of life scores. The WAMM study is expected to provide valuable insights into the potential of this novel approach for the treatment of MCL and could potentially lead to a paradigm shift in MCL therapy. The first results were presented at the 65th ASH Annual Meeting [1].
Could you just start by telling us a little bit about the unmet needs in MCL?
“MCL is a rare type of non-Hodgkin lymphoma and it is classified as an indolent disease, which means that it progresses slowly. Despite this, the vast majority of people with MCL do need some treatment. Traditionally, treatment for MCL has involved chemotherapy with rituximab, but these treatments are not always effective and can cause significant side effects. In addition, MCL is rarely curable, and most patients will relapse. Thus, there is a clear need for new and better treatments.”
What are some of the goals of the WAMM trial? How are you going about trying to fill that unmet need?
“The WAMM trial aims to evaluate the efficacy and safety of this novel treatment approach for MCL, involving a sandwich treatment model where acalabrutinib is given in a window before and after standard chemotherapy. The goal of this approach is to improve outcomes, combining the benefits of the novel agent with the proven efficacy of standard chemotherapy, while minimising side effects.”
What was the primary endpoint of the trial? Did the results meet your expectations?
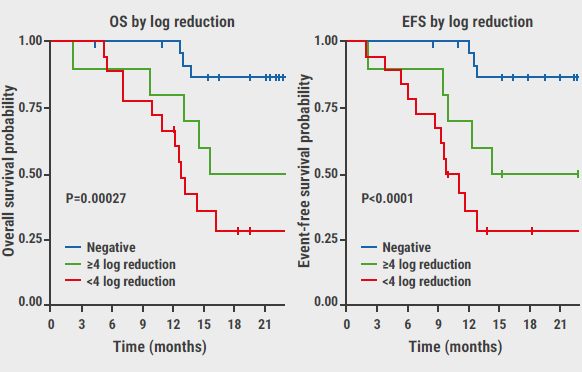
“The primary endpoint of the WAMM trial was the complete response rate (CRR) at the end of induction treatment. The CRR is a measure of how well the treatment has worked. A complete response is defined as the absence of any signs or symptoms of cancer. In the WAMM trial, the CRR was 88%, which is significantly higher than the CRR of 77% seen in historical data. These results were very encouraging and exceeded our expectations.”
The WAMM trial also evaluated the use of telehealth. What was the role of telehealth in the trial and how did it impact recruitment?
“The WAMM trial was conducted in Australia, which is a large country with a relatively small population. This made it difficult for participants to travel long distances to receive treatment. To address this challenge, the WAMM trial used a ‘hub-and-spoke’ telehealth model. The hub sites were responsible for delivering the more intensive aspects of the treatment, such as transplantation and chemotherapy. The spoke sites were responsible for providing local care and support to participants. This model allowed the trial to recruit patients from all over Australia, including rural and remote areas. It also allowed patients to receive treatment closer to home, which reduced their travel burden and improved their quality of life.”
Overall, what are your thoughts on the results of the WAMM trial?
“The first results of the WAMM trial are very promising. The sandwich treatment model with acalabrutinib showed excellent efficacy and safety. The telehealth hub-and-spoke model was also successful in facilitating patient recruitment and improving access to care for participants in rural and remote areas. I believe that these results have the potential to transform the treatment of MCL.”
- Hawkes E, et al. A Window Study of Acalabrutinib & Rituximab, Followed By Chemotherapy & Autograft (ASCT) in Fit Patients with Treatment Naïve Mantle Cell Lymphoma (MCL): First Report of the Investigator-Initiated Australasian Leukaemia & Lymphoma Group NHL33 ‘Wamm’ Trial. Abstract 735, 65th ASH Annual Meeting, 9–12 December 2023, San Diego, CA, USA.
- Wang ML, et al. N Engl J Med. 2022 Jun 30;386(26):2482-2494.
- Hermine O, et al. J Clin Oncol. 2023 Jan 20;41(3):479-484.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« ASH 2023 Highlights Podcast Next Article
Interview: Medicare’s first drug price negotiations: concerning? »
« ASH 2023 Highlights Podcast Next Article
Interview: Medicare’s first drug price negotiations: concerning? »
Table of Contents: ASH 2023
Featured articles
Meet the Trialist: Prof. Jeff Sharman on ELEVATE-TN
Leukaemia
FLT3-ITD-specific MRD assessment useful for clinical management of AML
MRD status rather than FLT3-ITD co-mutation is linked to the benefit of CR1-allo in NPM1-mutated AML
Promising results for quizartinib, venetoclax, and decitabine in FLT3-ITD mutated AML
AUGMENT-101: Excellent results for revumenib in R/R KMT2Ar leukaemia
Blinatumomab reduces toxicity in the consolidation phase in paediatric high-risk B-cell ALL
Promising results for olverembatinib in combination with venetoclax for Ph+ ALL
Undetectable MRD on maintenance venetoclax, acalabrutinib, and obinutuzumab in the majority of R/R CLL participants
Lymphoma
Is allogeneic stem cell transplantation a solid option in R/R LBCL or R/R T-cell lymphoma?
Encouraging results for the addition of acalabrutinib to lenalidomide and rituximab in follicular lymphoma
Can ibrutinib ameliorate outcomes in R/R ABC-DLBCL undergoing autoSCT?
Primary phase 2 efficacy and safety results of M-Pola in relapsed/refractory LBCL
SYMPATICO: Ibrutinib plus venetoclax boosts PFS in R/R mantle cell lymphoma
Multiple Myeloma
KdD outperforms Kd in R/R MM also in participants with poor renal function
IsKia: Novel treatment regimen for MM delivers high MRD-negativity rates
Novel standard-of-care in newly diagnosed MM
Myeloproliferative Neoplasms
TRANSFORM-1: High spleen volume reduction rates for navitoclax plus ruxolitinib in myelofibrosis
Momelotinib beats controls regarding transfusion outcomes in myelofibrosis
DALIAH: Peginterferon-α head-to-head against hydroxyurea in MPN
Non-Malignant Haematology
Long-term efficacy and safety of iptacopan in PNH with anaemia
ADVANCE IV: Swift responses on efgartigimod in ITP
Favourable QoL and bleeding outcomes for rilzabrutinib in ITP
Novel risk assessment model acts on increasing hospital-acquired venous thromboembolism rates among children
Miscellaneous Topics
Axatilimab may present a new therapeutic strategy in chronic GvHD
Pomalidomide may become the first approved therapy for hereditary haemorrhagic telangiectasia
Ancestry-specific study into CH delivers new leads
Featured Interviews
Interview: Sandwich treatment model shows promise for mantle cell lymphoma
Meet the Trialist: Prof. Jeff Sharman on ELEVATE-TN
Related Articles
February 3, 2022
Support for fourth COVID-19 jab in blood-cancer patients
August 28, 2020
Venetoclax + navitoclax promising for R/R ALL or LL
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

