https://doi.org/10.55788/a06f74c6
“Up to 82% of patients with PNH treated with anti-C5 have persistent anaemia, mostly due to extravascular haemolysis [1],” explained speaker Prof. Antonio Risitano (University of Naples, Italy). To overcome this issue, iptacopan, targeting factor B proximally in the alternative complement pathway, was tested in the APPLY-PNH trial (NCT04558918; n=97) [2]. Previous results showed that iptacopan monotherapy was superior to eculizumab/ravulizumab concerning various efficacy endpoints already after 24 weeks. Based on these results, participants in the anti-C5 therapy arm (n=35) switched to iptacopan (switch arm), whereas those already on iptacopan remained on this therapy. Prof. Risitano presented the 48-week findings of the APPLY-PNH trial [3].
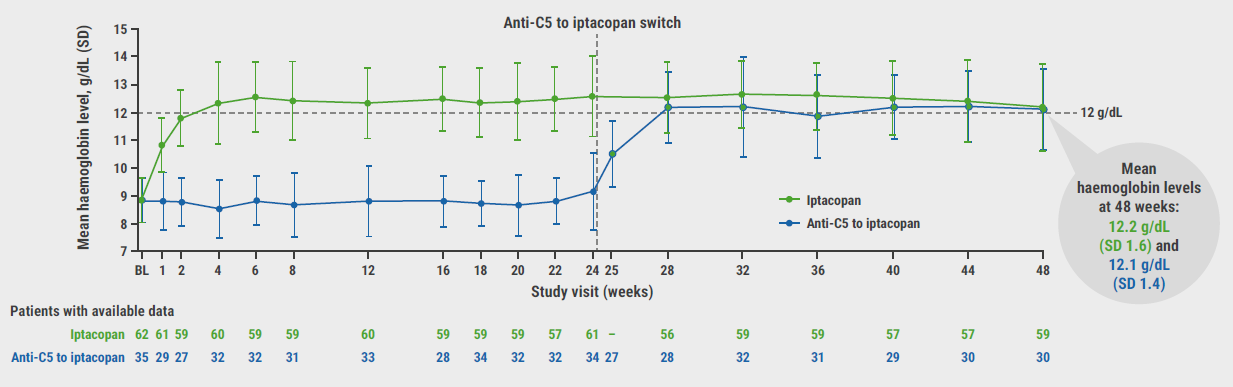
Improvements in haemoglobin levels were maintained in the iptacopan arm and participants who switched from anti-C5 to iptacopan (switch arm) displayed swift increases in haemoglobin levels (see Figure). Haemoglobin changes from baseline to week 47 were +3.35 g/dL and +3.36 g/dL in the iptacopan and switch arms, respectively. “Over 90% of participants in both iptacopan treatment arms did not require red blood cell transfusions after 2 weeks of iptacopan therapy,” expressed Prof. Risitano. Participant-reported fatigue, as measured by the FACIT-Fatigue score, was also improved at week 48, with changes from baseline of approximately +10 points in both iptacopan study arms. Breakthrough haemolysis events were uncommon in participants treated with iptacopan with rates of 3.2% and 8.2% in the first 24 weeks and final 24 weeks of the study in the iptacopan arm, and rates of 17.1% and 2.9% before and after the switch to iptacopan in the switch arm.
Figure: Mean haemoglobin levels increase upon iptacopan switch over the course of the APPLY-PNH study [3]
The safety profile of iptacopan monotherapy after 48 weeks was consistent with the favourable 24-week data. No serious infections, cases of treatment-emergent haemolysis, discontinuations due to treatment-related adverse events, or deaths were reported.
“This long-term data shows a durable response to iptacopan monotherapy with comprehensive control of intravascular and extravascular haemolysis,” concluded Prof. Risitano.
- Risitano AM, et al. Front Immunol.2019;10:1157
- Peffault de Latour R, et al. Blood 2022;140(Suppl 2):LBA-2
- Risitano AM, et al. Factor B inhibition with oral iptacopan mono therapy demonstrates sustained long-term efficacy and safety in anti-C5-treated patients with paroxysmal nocturnal hemoglobinuria and persistent anemia: final 48-week results from the multicenter, phase III APPLY-PNH trial. Abstract 571, 65th ASH Annual Meeting, 9–12 December 2023, San Diego, CA, USA.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« ADVANCE IV: Swift responses on efgartigimod in ITP Next Article
DALIAH: Peginterferon-α head-to-head against hydroxyurea in MPN »
« ADVANCE IV: Swift responses on efgartigimod in ITP Next Article
DALIAH: Peginterferon-α head-to-head against hydroxyurea in MPN »
Table of Contents: ASH 2023
Featured articles
Meet the Trialist: Prof. Jeff Sharman on ELEVATE-TN
Leukaemia
FLT3-ITD-specific MRD assessment useful for clinical management of AML
MRD status rather than FLT3-ITD co-mutation is linked to the benefit of CR1-allo in NPM1-mutated AML
Promising results for quizartinib, venetoclax, and decitabine in FLT3-ITD mutated AML
AUGMENT-101: Excellent results for revumenib in R/R KMT2Ar leukaemia
Blinatumomab reduces toxicity in the consolidation phase in paediatric high-risk B-cell ALL
Promising results for olverembatinib in combination with venetoclax for Ph+ ALL
Undetectable MRD on maintenance venetoclax, acalabrutinib, and obinutuzumab in the majority of R/R CLL participants
Lymphoma
Is allogeneic stem cell transplantation a solid option in R/R LBCL or R/R T-cell lymphoma?
Encouraging results for the addition of acalabrutinib to lenalidomide and rituximab in follicular lymphoma
Can ibrutinib ameliorate outcomes in R/R ABC-DLBCL undergoing autoSCT?
Primary phase 2 efficacy and safety results of M-Pola in relapsed/refractory LBCL
SYMPATICO: Ibrutinib plus venetoclax boosts PFS in R/R mantle cell lymphoma
Multiple Myeloma
KdD outperforms Kd in R/R MM also in participants with poor renal function
IsKia: Novel treatment regimen for MM delivers high MRD-negativity rates
Novel standard-of-care in newly diagnosed MM
Myeloproliferative Neoplasms
TRANSFORM-1: High spleen volume reduction rates for navitoclax plus ruxolitinib in myelofibrosis
Momelotinib beats controls regarding transfusion outcomes in myelofibrosis
DALIAH: Peginterferon-α head-to-head against hydroxyurea in MPN
Non-Malignant Haematology
Long-term efficacy and safety of iptacopan in PNH with anaemia
ADVANCE IV: Swift responses on efgartigimod in ITP
Favourable QoL and bleeding outcomes for rilzabrutinib in ITP
Novel risk assessment model acts on increasing hospital-acquired venous thromboembolism rates among children
Miscellaneous Topics
Axatilimab may present a new therapeutic strategy in chronic GvHD
Pomalidomide may become the first approved therapy for hereditary haemorrhagic telangiectasia
Ancestry-specific study into CH delivers new leads
Featured Interviews
Interview: Sandwich treatment model shows promise for mantle cell lymphoma
Meet the Trialist: Prof. Jeff Sharman on ELEVATE-TN
Related Articles
February 4, 2022
Improved risk stratification in MDS via gene-based scoring system
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

