VARSITY was the first head-to-head trial comparing the efficacy and safety of biologics in patients with moderately to severely active UC, and was one of the highlights of the 2019 edition of the ECCO-IBD meeting [2,3]. To assess QoL, the IBDQ was used. Clinically meaningful IBDQ improvement was defined as an increase in total score of ≥16 points from baseline to week 52; IBDQ remission was a total score of >170 points at week 52.
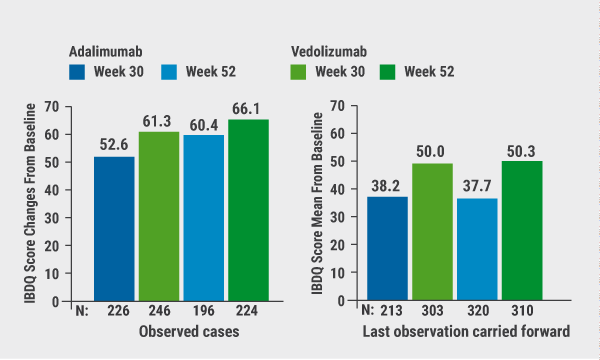
Results showed a clinically meaningful IBDQ improvement at week 52 in 199/383 patients (52.0%) versus 163/386 (42%) in the vedolizumab and adalimumab group, respectively (treatment difference 9.7%). IBDQ remission was achieved by 192 (50%) and 156 (40%) patients, respectively (treatment difference 9.6%). Mean changes in IBDQ total score from baseline for observed cases favoured vedolizumab over adalimumab (see Figure). IBDQ subscores showed similar favourable trends for vedolizumab. Reduced inflammation, as indicated by improvements in C-reactive protein (CRP) and faecal calprotectin (FCP), was consistent with improvements in QoL.
Figure. IBDQ total score changes from baseline at weeks 30 and 52 (full analysis set) [1]
Using efficacy data of the VARSITY trial, another study compared the cost-effectiveness of vedolizumab and adalimumab [4]. The clinical foundation of this model is predicated primarily on head-to-head evidence from phase 3 trials. Modelled outcomes indicated that UC patients are likely to achieve better clinical outcomes, as vedolizumab was associated with higher induction response (52.9% vs 45.1%) and remission rates (22.0% vs 14.4%). Also, based on US payer costings, lower direct medical costs are associated with vedolizumab (USD $90,673) than with adalimumab (USD $137,007), though this may not be applicable to other countries.
- Loftus EV, et al. ECCO-IBD 2020, DOP24.
- Schreiber S, et al. ECCO-IBD 2019, OP34.
- Sands BE, et al. N Engl J Med. 2019 Sep 26;381(13):1215-1226.
- Schultz R, et al. ECCO-IBD 2020, P607.
Posted on
Previous Article
« Vedolizumab and anti-TNF therapies: a real-world comparison Next Article
Glyco-fingerprint as risk factor of UC-associated cancer »
« Vedolizumab and anti-TNF therapies: a real-world comparison Next Article
Glyco-fingerprint as risk factor of UC-associated cancer »
Table of Contents: ECCO 2020
Featured articles
Gut Microbiome as Treatment Target
Response to faecal microbiota transplantation in UC
Bioactives produced by gut bacteria to modulate immune response
Big Data Analysis
Multi-omics help describe CD phenotypes
The positive impact of genetic data on drug development
Experimental Therapies: Study Results
AMT-101: an oral human IL-10 fusion protein
Phase 2 results of first-in-class TL1A inhibitor
Open-label extension study of risankizumab: final results
Clinical remission after dose escalation of upadacitinib
Short- and Long-Term Treatment Results
Infliximab discontinuation increases relapse risk
Tofacitinib ‘real-world’ effectiveness in active UC
Subcutaneous ustekinumab as maintenance therapy in UC
Subcutaneous vedolizumab maintenance therapy in CD
Vedolizumab treatment persistence and safety
Specific Therapeutic Strategies
Impact of strategies on intestinal resection rate
Early ileocaecal resection in CD patients failing conventional treatment
Biologics before surgery in IBD do not elevate infection risk
Top-down infliximab superior to step-up in children with CD
High versus standard adalimumab in active UC
Head-to-Head Comparison of Treatments
Vedolizumab and anti-TNF therapies: a real-world comparison
Cancer Risk
Increased risk of small bowel cancer in IBD
Increased incidence of colorectal cancer and death in CD
Risk of rectal, anal cancer increased in perianal CD
Glyco-fingerprint as risk factor of UC-associated cancer
Miscellaneous Topics
Resolution of mucosal inflammation has dramatic effect
PICaSSO validated in real-life study
Re-inducing inflammation in organoids from UC patients
Role of immune cells in intestinal fibrosis
Association between meat consumption and IBD risk
CD exclusion diet corrects dysbiosis
Related Articles

April 14, 2020
Impact of biologicals on faecal microbiota
April 14, 2020
PICaSSO validated in real-life study
April 14, 2020
Possible new treatment targets in IBD
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

