The heart is affected by COVID-19 infection through different mechanisms. Most relevant from a pathological point of view is myocardial damage due to generalised inflammatory reactions and cytokine storms.
“We found that cardiac patients are at increased risk for complications [of a COVID-19 infection] and mortality,” said Prof. Marco Metra (University of Brescia, Italy) on his early experiences during the COVID-19 pandemic in Northern Italy [1]. Prof. Metra and his colleagues published a study including 99 patients with COVID-19 pneumonia. They compared 53 patients with a history of cardiac disease with 46 patients without this comorbidity. Mortality was dramatically higher in patients with cardiac disease compared with the others (36% vs 15%, log-rank P=0.019; relative risk 2.35; 95% CI 1.08–5.09). In addition, cardiac patients had a significantly higher rate of thromboembolic events and septic shock during hospitalisation (23% vs 6% and 11% vs 0%, respectively; see Figure 1) [2].
Figure 1: Major complications and deaths at 14 days. Modified from [2]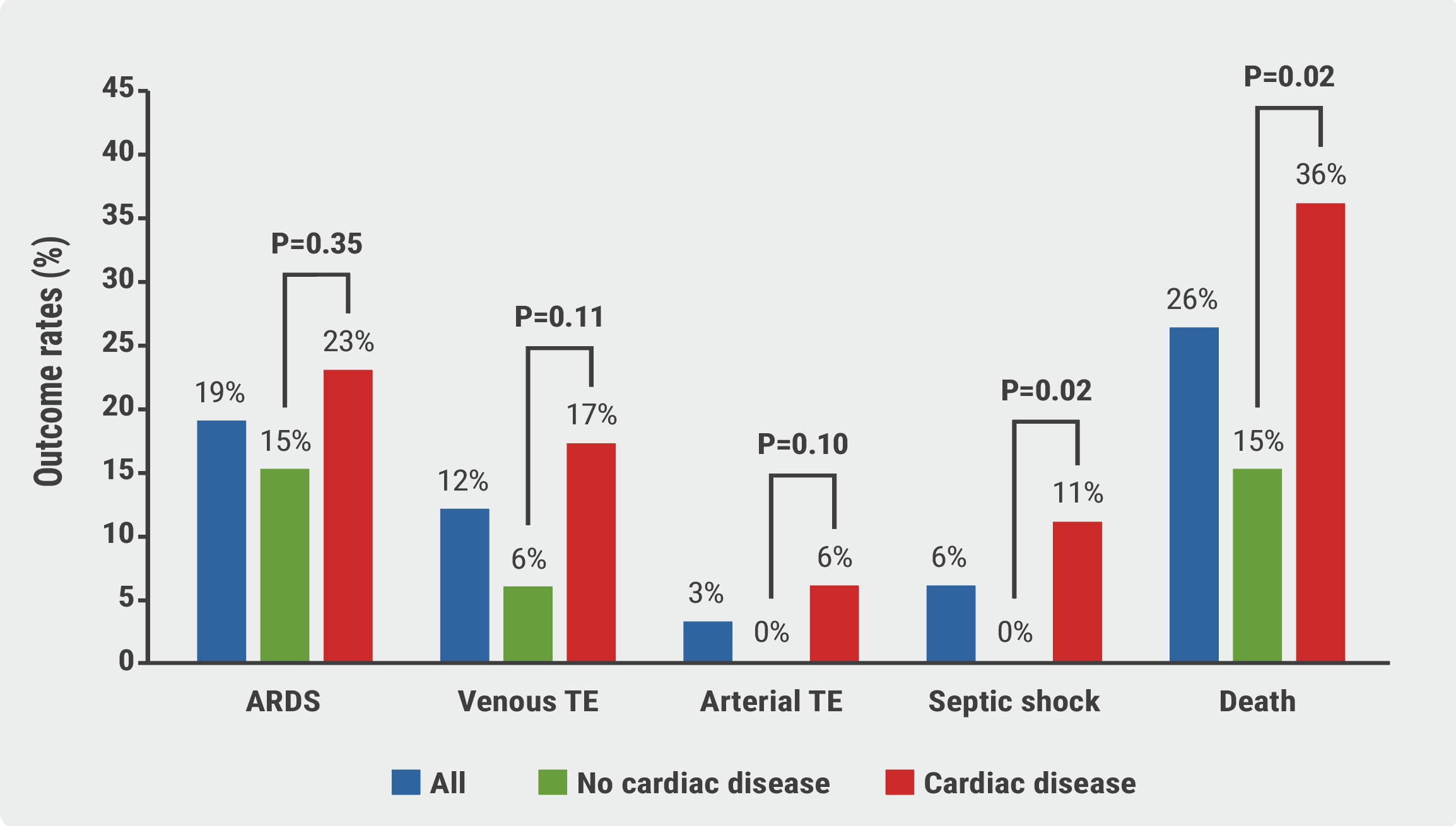 ARDS, acute respiratory distress syndrome; TE, thromboembolism
ARDS, acute respiratory distress syndrome; TE, thromboembolism
Elevated troponin: a negative prognostic factor
Prof. Metra also pointed out that elevated high-sensitive troponin T levels have been associated with a worse outcome. This finding was corroborated in a multicentre study including 614 patients with laboratory-confirmed COVID-19 who were hospitalised in 13 cardiology units in Italy [3]. In this study, elevated troponin was an independent variable associated with in-hospital mortality and a greater risk of cardiovascular and non-cardiovascular hospitalisation. “If a patient has low troponin and low B-type natriuretic peptide levels, they have a good prognosis,” Prof. Metra said.
In an imaging study, patterns of myocardial injury in recovered troponin-positive COVID-19 patients were assessed by cardiovascular magnetic resonance (CMR) [4]. All participants (n=148) had suffered from severe COVID-19 infections requiring hospital admission. CMR imaging was performed at a median of 68 days. Normal cardiac imaging findings were found for 54% of patients. In 32%, a scar pattern was detected consistent with inflammatory myocardial damage. In 28% of patients, an ischaemic pattern was noted, of which two-thirds had no previous history. Other studies have also revealed that a substantial percentage of patients show abnormal CMR findings after acute COVID-19 – even after mild disease. “We have patients with pathologic CMR findings but normal left ventricular function,” Prof. Metra said. Abnormalities of myocardial tissue characterised by MRI are common during COVID-19 recovery, but causal relationships of these tissue changes to symptoms and future cardiac events are not yet known [5]. Symptomatic COVID-19 infections can result in acute and chronic sequelae for the heart.
Prof. Metra pointed out that there are 2 main mechanisms of injury in COVID-19: myocardial ischaemia and myocardial inflammation. The latter is caused mainly by systemic inflammation but also by direct viral injury. Myocardial ischaemia is accompanied by increased myocardial oxygen demand, systemic hypoxia, endotheliitis, plaque rupture, and thrombosis.
Microthrombi: a major cause of cardiac injury in COVID-19
“We know that COVID-19 is specifically associated with endothelial and vascular thrombosis,” Prof. Metra explained. A pathological analysis of 40 hearts from hospitalised patients who died of COVID-19 in Bergamo, Italy, showed that microthrombi were the most common pathological cause of myocyte necrosis [6]. Microthrombi had significantly greater fibrin and terminal complement C5b-9 immunostaining compared with intramyocardial thromboemboli from COVID-19-negative subjects. Non-occlusive fibrin microthrombi without universal acute ischaemic injury were also found in a pathological case series of patients who died from COVID-19 [7].
Prof. Metra also indicated that the virus itself only attacks the heart in rare cases, although this has been observed in a patient with cardiogenic shock [8]. More often, an adverse effect is mediated by macrophages, as was shown in an international, multicentre study, where cardiac tissues from the autopsies of 21 COVID-19 patients were assessed by cardiovascular pathologists [9]. The inflammatory cell composition by immunohistochemistry was assessed. Increased interstitial macrophage infiltration was present in 86% of the cases and multifocal lymphocytic myocarditis in a small fraction of the cases.
Taken together, inflammation-induced cardiac damage and necrosis are histologic hallmarks of cardiac involvement in COVID-19. Perivascular or vascular inflammation and/or macrophages are often present. In some cases, mild or absent inflammatory infiltrate with lymphopaenia is seen. “The most frequent scenario is myocardial damage due to generalised inflammatory reactions and cytokine storms,” Prof. Metra concluded.
- Metra M. COVID-19 and cardiorespiratory failure – from pathophysiology to clinical practice. Heart Failure and World Congress on Acute Heart Failure 2021, 29 June–1 July.
- Inciardi RM, et al. Eur Heart J 2020;41:1821–9.
- Lombardi CM, et al. JAMA Cardiol 2020;5:1274–80.
- Kotecha T, et al. Eur Heart J 2021;42:1866–78.
- Friedrich MG, Cooper LT. Eur Heart J 2021;42:1879–82.
- Pellegrini D, et al. Circulation 2021;143:1031–42.
- Bols MC, et al. Circulation 2021;143:230–43.
- Tavazzi G, et al. Eur J Heart Fail 2020;22:911–5.
- Basso C, et al. Eur Heart J 2020;41:3827–35.
Copyright ©2021 Medicom Medical Publishers
Posted on
« Increased COVID-19 mortality in patients with cardiorenal comorbidity Next Article
Telemedicine: Every light has its shadow »
Table of Contents: HFA 2021
Featured articles
Inconclusive results for dapagliflozin treatment in heart failure
Late-Breaking Trials
Iron substitution improves LVEF in intensively treated CRT patients with iron deficiency
Novel mineralocorticoid receptor antagonist effective irrespective of HF history
Iron substitution in iron-deficient HF patients is highly cost-effective
Omecamtiv mecarbil might be less effective in patients with atrial fibrillation or flutter
Vericiguat effective irrespective of atrial fibrillation status
Baroreflex activation: a novel option to improve heart failure symptoms
Beta-blocker withdrawal to enhance exercise capacity in heart failure?
Inconclusive results for dapagliflozin treatment in heart failure
Computerised cognitive training improves cognitive function in HF patients
COVID-19 and the Heart
COVID-19-related HF: from systemic infection to cardiac inflammation
Myocardial infarction outcomes were significantly affected by the pandemic
TAPSE effective biomarker associated with high-risk of severe COVID-19
COVID-19 in AF patients with HF: no higher mortality but longer hospital stay
Cancer and the Heart
Heart failure patients might be at an increased risk for head and neck cancer
Trastuzumab associated with cardiotoxicity in breast cancer
Heart Failure Prevention and HRQoL in the 21st century
Psychoactive substances put young people at risk of cardiovascular disease
The challenge of improving the quality of life of heart failure patients
SGLT2 Inhibitors in Heart Failure
Empagliflozin linked to lower cardiovascular risk and renal events in real-world study
Efficacy of dapagliflozin and empagliflozin not influenced by diabetes status
Biomarker panel predicts SGLT2 inhibitor response
Best of the Posters
Real-world study suggests sacubitril/valsartan benefits elderly patients with HF
Proenkephalin: A useful biomarker for new-onset heart failure?
Weight loss associated with increased mortality risk in heart failure patients
Echocardiographic parameters linked to dementia diagnosis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com


