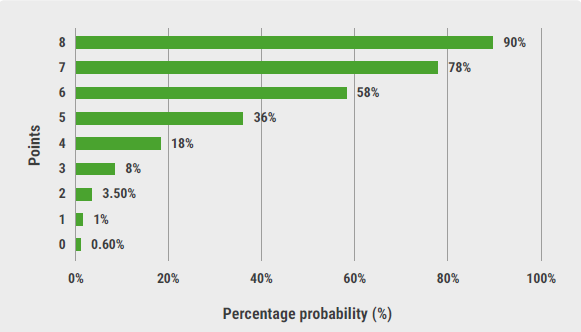
With a median follow-up of 30.6 months, KEYNOTE-426 demonstrated that pembrolizumab and axitinib (n=432) had a significant benefit compared with sunitinib (n=429) in mRCC patients in terms of overall survival (HR 0.68; 95% CI 0.55-0.85), progression-free survival (HR 0.71; 95% CI 0.60-0.84), and objective response rate (60% vs 40%). Key endpoints regarding the patient-reported outcome analyses included time to deterioration and change from baseline over time. The primary analysis timepoint was 30 weeks. Assessment of time to deterioration was continued up to week 90.
The patient-reported outcome assessment was performed with the following instruments, although arm-specific adjustments to the treatment protocol changed the scheduling between the arms:
- QLQ-C30: general QoL in cancer patients;
- FKSI-DRS: 9 kidney cancer-specific cancer-related symptoms; and
- EQ-5D-3L VAS: general state of health.
The primary outcome –change from baseline over time– never met the threshold for minimally important differences between the 2 study arms at any point during the 30 weeks examined, with any of the validated instruments. Thus, the researchers concluded that health-related QoL in patients treated with the combination pembrolizumab and axitinib was similar to those receiving sunitinib monotherapy. There were also no differences between the treatment groups in time to deterioration in the confirmed analysis (HR 1.12; 95% CI 0.91-1.38), as well as in the unconfirmed analysis (HR 1.02; 95% CI 0.86-1.20).
- Bedke J, et al. EAU20 Virtual Congress, 17-26 July 2020, Game-changing Session 4.
Posted on
Previous Article
« Debate: upfront cytoreductive nephrectomy or not? Next Article
Understanding MIBC biology for novel treatment options »
« Debate: upfront cytoreductive nephrectomy or not? Next Article
Understanding MIBC biology for novel treatment options »
Table of Contents: EAU 2020
Featured articles
Surgical Techniques and Safety
The new adjustable artificial sphincter victo: Surgical technique and results after a follow-up of more than one year
New urosepsis data from the SERPENS study
Stones
Intra-operative cone-beam computed tomography for detecting residual stones in percutaneous nephrolithotomy
Pressure and temperature: do high-power lasers pose a threat?
Radiation stewardship for patient and endourologist
New lithotripter data: improved stone clearance
Renal Cancer
Beyond the limits of ultrasound: Three dimensional augmented reality robot assisted partial nephrectomy (3D AR-RAPN) for complex renal masses
Imaging guided surgery with augmented reality for robotic partial nephrectomy
KEYNOTE-426: no QoL differences pembrolizumab + axitinib versus sunitinib
Debate: upfront cytoreductive nephrectomy or not?
Robotic-assisted partial nephrectomy: lower morbidity
Bladder Cancer
Reduced BCG frequency, faster NMIBC recurrence
Nadofaragene firadenovec effective in BCG-unresponsive papillary NMIBC
Understanding MIBC biology for novel treatment options
Prostate Cancer & Imaging
Transperineal laser ablation of prostate
Prostatectomy: R-LRPE better than LRPE for continence
PSMA PET-CT staging is 27% more accurate
Docetaxel + hormonal therapy: improved prostate cancer PFS
ARAMIS subgroup analysis: darolutamide benefits across PSADT groups
Large patient-driven survey reveals QoL issues after prostate cancer treatment
Targeting steroid sulphatase in resistant prostate cancer cells
Good tolerance of post-RP radiotherapy ± short-term ADT
BPH & LUTS
Minimizing post-operative stress urinary incontinence after HoLEP: Our preliminary experience and short-term results of ‘’En Bloc’’ technique with early apical release
LUTS 2-year outcomes: aquablation versus TURP
HoLEP versus PVP in prospective randomised trial
Testis Cancer & Andrology
Peyronie’s disease: surgical options
Infertility and testis cancer risk: causal or association?
32% more men complain of reduced sex drive in 2019 versus 2009
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com