Testicular germ cell tumours (TGCTs) display a tremendous variety of morphologic elements, often in mixed pathologies. Generally, TGCTs are classified as either germ cell neoplasia in situ (GCNIS), or non-GCNIS-associated TGCTs. Most malignant TGCTs originate from GCNIS. Molecular and genetic studies have highlighted 7 types of TGCTs, which can also be understood within terms of developmental processes.
Diagnosis, treating, and follow-up of TGCTs relies on understanding of the biological drivers of the disease, yet little is really known. TGCTs originate from GCNIS precursors, which are characterised by expression of the markers OCT4, SOX17, NANOG, as well as TSPY, and cKIT and its ligand KITLG. Differentiation is regulated by gene-environment factors and stromal cell interactions. When OCT4 no longer represses AKT transcription, totipotency survival can promote polyploidisation, which is considered an early event in the formation of GCNIS, and ultimately lead to TGCTs (see Figure) [2].
Figure: Proposed model of TGCT development from GCNIS precursors [2]
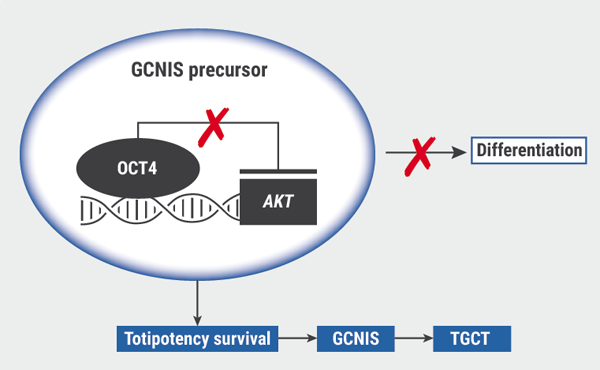
GCNIS, germ cell neoplasia in situ; TGCT, testicular germ cell tumours.
Adapted from Cheng et al. 2018.
In patients with TGCTs, fertility preservation is an important component following cancer treatment, and the question posed here is whether infertility and TGCTs are causal or associated [1]. What we do know is that the GCNIS differentiation block of the spermatogonial stem cell is the earliest pathogenetic link between testicular carcinogenesis and infertility. The unique synchronised duplication using syncytia (=intercellular bridges) are characteristic of this spermatogonial stem cell compartment and there has been some speculation on the stability of those syncytia in promoting TGCTs and possibly fertility, which might be attributable to microtubule or centrosome stability at those sites. The cells would multiply at an undifferentiated state, and would not produce mature sperm in those instances.
Although a large number of studies support an association between infertility and TGCTs, causality has proven difficult to pin down. For example, a large and recent retrospective cohort study concluded that there was an increased risk of TGCT development in 20,433 men who had undergone semen analysis compared with 20,433 age- and birth year-matched fertile controls. In particular, men with oligospermia based on low concentration (HR 11.9; 95% CI 4.9–28.8) or low sperm count (HR 10.3; 95% CI 4.1–26.2) and infertile men with normozoospermia (HR 2.9; 95% CI 1.2–6.7) had an increased risk of testicular cancer [3].
Really novel in the field is that the microRNA miR-371a-3p is a putative molecular biomarker for TGCTs. The suspected mechanism is that all members of the miR-371-3 family inactivate P53, thereby disrupting cellular senescence. Furthermore, miR-371a-3p is expressed explicitly in all malignant components of TGCTs, either being type I or II, as well as being detectable in serum, plasma, and cerebrospinal fluids of patients with malignant TGCTs. There have been some reports that miR-371a-3p can serve as a robust molecular liquid biopsy TGCT biomarker, and is more informative compared with the trusted markers alpha fetoprotein (AFP) and/or the beta subunit of human chorionic gonadotropin (β-hCG). Prof. Looijenga concluded that there is no effective way at the moment to tease out the cause and effect currently; the complex effects of surgery, chemotherapy, and radiotherapy may contribute to infertility, particularly in those at elevated risk of baseline subfertility or infertility, but it is essential to assume that there may be connected aspects of these 2 conditions. The microenvironment may also be relevant, but reliable markers are still being developed to assay those effects.
- Looijenga LH, et al. EAU20 Virtual Congress, 17-26 July 2020, State-of-the-art-lecture.
- Cheng L, et al. Nat Rev Dis Primers 2018;4, 29.
- Hanson HA, et al. Fertil Steril. 2015;105(2):322-8.e1.
Posted on
Previous Article
« 32% more men complain of reduced sex drive in 2019 versus 2009 Next Article
Peyronie’s disease: surgical options »
« 32% more men complain of reduced sex drive in 2019 versus 2009 Next Article
Peyronie’s disease: surgical options »
Table of Contents: EAU 2020
Featured articles
Surgical Techniques and Safety
The new adjustable artificial sphincter victo: Surgical technique and results after a follow-up of more than one year
New urosepsis data from the SERPENS study
Stones
Intra-operative cone-beam computed tomography for detecting residual stones in percutaneous nephrolithotomy
Pressure and temperature: do high-power lasers pose a threat?
Radiation stewardship for patient and endourologist
New lithotripter data: improved stone clearance
Renal Cancer
Beyond the limits of ultrasound: Three dimensional augmented reality robot assisted partial nephrectomy (3D AR-RAPN) for complex renal masses
Imaging guided surgery with augmented reality for robotic partial nephrectomy
KEYNOTE-426: no QoL differences pembrolizumab + axitinib versus sunitinib
Debate: upfront cytoreductive nephrectomy or not?
Robotic-assisted partial nephrectomy: lower morbidity
Bladder Cancer
Reduced BCG frequency, faster NMIBC recurrence
Nadofaragene firadenovec effective in BCG-unresponsive papillary NMIBC
Understanding MIBC biology for novel treatment options
Prostate Cancer & Imaging
Transperineal laser ablation of prostate
Prostatectomy: R-LRPE better than LRPE for continence
PSMA PET-CT staging is 27% more accurate
Docetaxel + hormonal therapy: improved prostate cancer PFS
ARAMIS subgroup analysis: darolutamide benefits across PSADT groups
Large patient-driven survey reveals QoL issues after prostate cancer treatment
Targeting steroid sulphatase in resistant prostate cancer cells
Good tolerance of post-RP radiotherapy ± short-term ADT
BPH & LUTS
Minimizing post-operative stress urinary incontinence after HoLEP: Our preliminary experience and short-term results of ‘’En Bloc’’ technique with early apical release
LUTS 2-year outcomes: aquablation versus TURP
HoLEP versus PVP in prospective randomised trial
Testis Cancer & Andrology
Peyronie’s disease: surgical options
Infertility and testis cancer risk: causal or association?
32% more men complain of reduced sex drive in 2019 versus 2009
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

