MAS is a rare, systemic life-threatening disorder characterised by uncontrolled hyperinflammation which may develop on a background of rheumatic diseases such as SJIA. It is classified as a secondary form of hemophagocytic lymphohistiocytosis (HLH) and is caused by excessive activation and expansion of T cells and macrophages. In recent years, evidence has shown uncontrolled overproduction of IFNγ as a major driver of hyperinflammation and hypercytokinaemia in diseases such as MAS and HLH. Emapalumab is a monoclonal antibody that binds to and neutralises interferon gamma (IFNγ).
Dr Fabrizio De Benedetti et al. (Ospedale Pediatrico Bambino Gesù, Italy) initiated a pilot study which aimed to assess the pharmacokinetics, efficacy, and safety of intravenous emapalumab in patients with MAS, and to confirm the proposed dose regimen. A total of 6 patients who had MAS, confirmed or high presumption of SJIA, and inadequate response to high-dose intravenous glucocorticoids were included in the study. Five of them were female, the median age was 11 years with range 2-25 years. Prior to emapalumab, all patients failed methylprednisolone pulse therapy. Four patients received concomitantly either cyclosporine A (n=2) and/or cyclosporine A and anakinra (n=2), a human IL-1-receptor antagonist. All subjects received an initial intravenous emapalumab dose of 6 mg/kg. Treatment was continued at 3 mg/kg twice weekly, for a total of ≤4 weeks, depending on achieved response rate. Serum concentrations of emapalumab, as well as IFNγ-induced chemokine CXCL9 and sIL2R, were measured. Efficacy was defined as a complete response by week 8; absence of MAS signs and white blood cells and platelets counts above lower limit of normal; lactate dehydrogenase (LDH)/AST/ALT below 1.5x upper limit of normal, fibrinogen >100 mg/dL, and ferritin decreased by ≥80% or to <2,000 ng/mL, whichever was lower).
The trial showed that treatment with emapalumab led to rapid neutralisation of IFNγ and that a complete response was reached in all patients by week 8. All patients weaned from glucocorticoids. A cytomegalovirus reactivation was reported as a serious event possibly related to emapalumab, but resolved completely with treatment. It was concluded that emapalumab treatment was effective and safe in controlling MAS. Thus, translational therapeutics in the MAS arena is pointing towards a key role for IFNγ in addition to other studies showing key roles for IL-18 and possibly IL-1, which is helping to elucidate the immunopathogenetic basis for these diseases.
- De Benedetti, F. et al. Abstract OP0204. EULAR 2019
Posted on
Previous Article
« Peficitinib likely efficacious and safe Next Article
Clinical effectiveness of fenebrutinib in RA patients with methotrexate or TNFi failure »
« Peficitinib likely efficacious and safe Next Article
Clinical effectiveness of fenebrutinib in RA patients with methotrexate or TNFi failure »
Table of Contents: EULAR 2019
Featured articles
Efficacy and safety of ixekizumab versus adalimumab in patients with PsA
Rheumatoid Arthritis
Cohort study shows improvement during 25 years of RA treatment
Filgotinib in RA patients with inadequate response or naïve to methotrexate
Clinical effectiveness of fenebrutinib in RA patients with methotrexate or TNFi failure
Short methotrexate stop is safe in patients with RA
Tofacitinib is safe according to real-world data analysis
Tapering of prednisone in RA patients who achieved low disease activity or remission with tocilizumab
Efficacy and safety of E6011 in RA patients with inadequate response to methotrexate
Preliminary efficacy and safety data of RG6125 in RA patients with an inadequate response to TNF inhibitors
Integrated 10-year analysis confirms safety profile abatacept
Switching among multiple infliximab biosimilars does not cause immunogenicity
Switch to sarilumab from adalimumab is efficacious and safe
Axial Spondyloarthritis
Treat-to-target approach emerging in axial spondyloarthritis
NSAIDs consumption is linked to patient-assessed disease activity and decreases with use of TNF inhibitors
Psoriatic Arthritis
Efficacy and safety of ixekizumab versus adalimumab in patients with PsA
Efficacy and safety of bimekizumab in patients with active PsA
Filgotinib is efficacious and safe in PsA
Ixekizumab improves signs and symptoms in TNFi-naïve PsA patients
Etanercept and methotrexate as first-line treatment in PsA
Unacceptable pain is common in patients with psoriatic arthritis
Osteoarthritis and Osteoporosis
Miscellaneous
Interstitial lung disease in rheumatic diseases and systemic sclerosis
Emapalumab in patients with macrophage activation syndrome
Support for tocilizumab use in giant cell arteritis
Related Articles
September 4, 2019
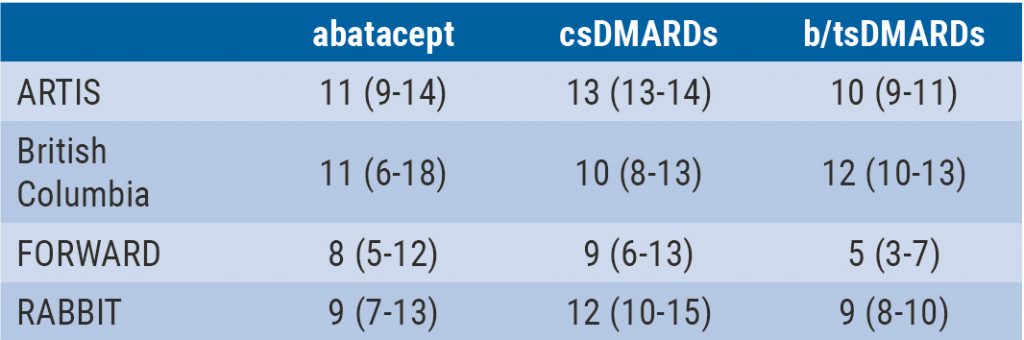
Integrated 10-year analysis confirms safety profile abatacept
September 4, 2019
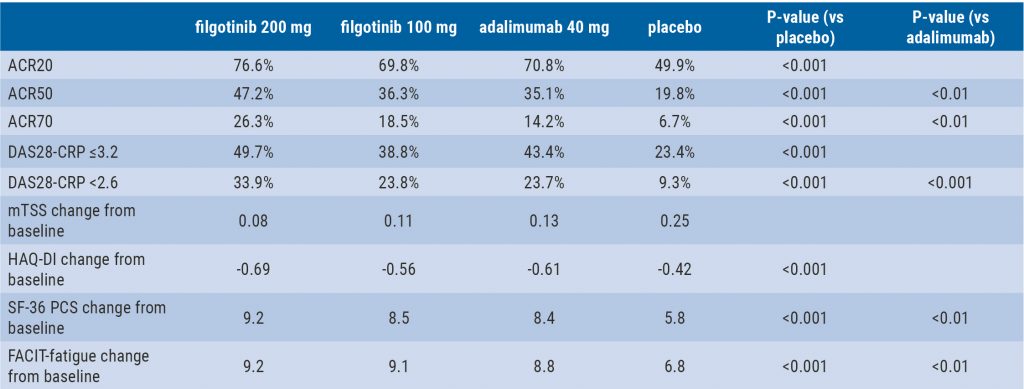
Treat-to-target approach emerging in axial spondyloarthritis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

