Most recommended treatments in the EuroGuiDerm guidelines are quite familiar [1]. “We still have the traditional treatments (i.e. acitretin, ciclosporin, fumarates, and methotrexate) as first-line. Some patients bypass the first-line treatments, going directly into other treatment options, especially in severe cases if treatment success cannot be expected with the conventional drugs,” Prof. Nast explained.
Efficacy compared
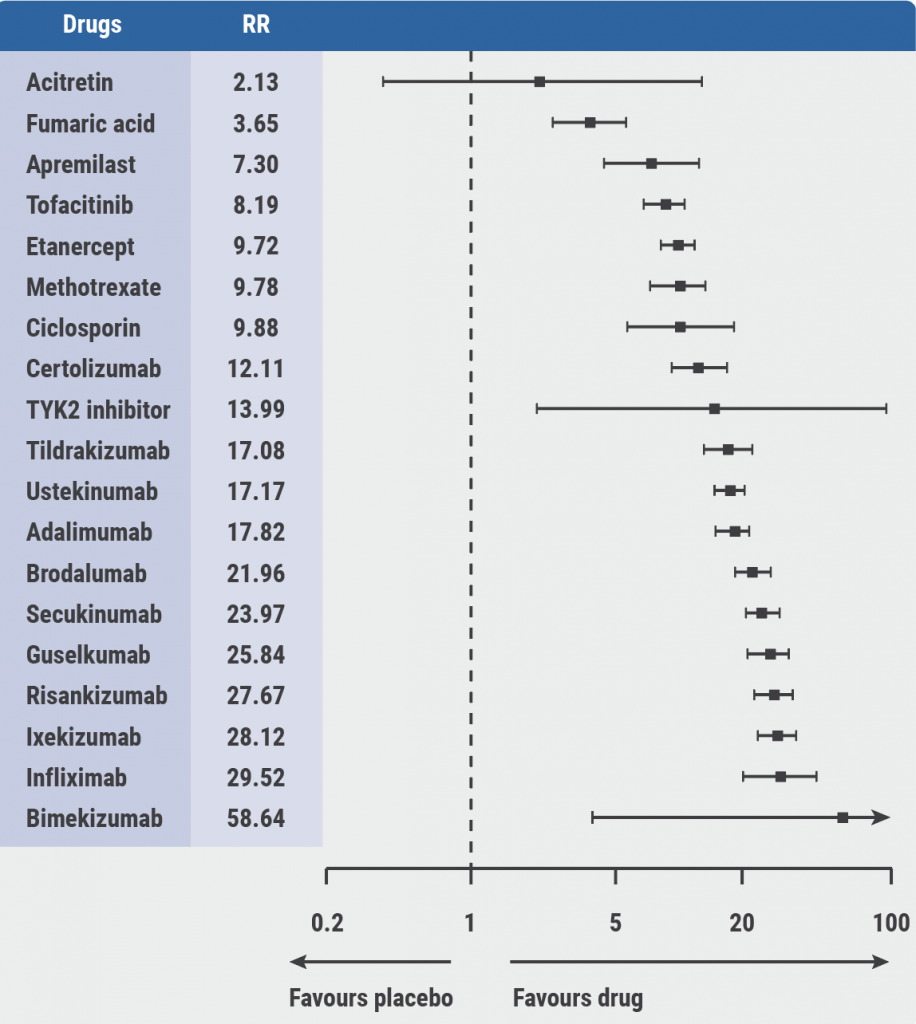
A network meta-analysis, which was used as an evidence basis for the guidelines, provided estimates of all pairwise intervention comparisons that are connected within a network, including those that have never been directly compared in randomised controlled trials, the latter being referred to as indirect evidence [3]. “There are some pronounced differences in the efficacy,” Prof. Nast mentioned (see Figure). Safety is more difficult to compare because different side effects cannot be grouped easily.
Figure: PASI 90: Relative effects from the network meta-analysis against placebo [3]
The guidelines contain detailed advice on the use of each individual drug. Important factors to consider are lab controls, adverse drug reactions, special considerations during treatment, contraindications, and drug interactions.
Management of PsA
How should patients with psoriasis and concomitant psoriatic arthritis (PsA) be managed? The guidelines recommend starting a conventional synthetic DMARD (preferably methotrexate) early to prevent disease progression and erosive destruction of joints for patients with moderate-to-severe psoriasis and peripheral active joint involvement despite the usage of NSAIDs. Alternatively, glucocorticoid site injections in case of poor prognosis due to polyarthritis, increased inflammatory markers and erosive changes, and extra-articular musculoskeletal manifestations.
For inadequately responding patients after ≥1 synthetic DMARD, the use of biological DMARDs is recommended as monotherapy or in combination with synthetic DMARDs. Concerning efficacy for patients with PsA, a subsequent systematic review was published [4]. “The differences are much less pronounced compared with the treatment of plaque-type psoriasis,” Prof. Nast explained. Taken together in a decision aid, methotrexate is first-line treatment of psoriasis with joint involvement. “If this does not work, biologics should be considered next.”
Management of comorbid IBD
How should patients with psoriasis with comorbid inflammatory bowel disease (IBD) be managed? “This is a patient group where we have to be careful,” Prof. Nast stressed. IL-17 blockers are not strongly recommended. Ustekinumab is a better option. The choice after that is anti-IL-23. “If you need a cheaper option or an oral treatment, we also give guidance on that, with methotrexate being preferred for Crohn’s disease and ciclosporin for ulcerative colitis,” Prof. Nast concluded.
- Living EuroGuiDerm Guideline for the systemic treatment of psoriasis vulgaris. July 2020. https://www.edf.one/home/Guidelines/EuroGuiDerm-psoriasis-vulgaris.html
- Nast A. Treatment guidelines/best practice psoriasis. 6th World Psoriasis & Psoriatic Arthritis Conference, 30 June–3 July 2021.
- Sbidian E, et al. Cochrane Database Syst Rev. 2020;1:CD011535.
- Dressler C, et al. J Eur Acad Dermatol Venereol. 2019;33:1249-60.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Psoriatic arthritis: Guidelines and best practice Next Article
Whole-exome sequencing to study the underlying pathogenesis of psoriasis »
« Psoriatic arthritis: Guidelines and best practice Next Article
Whole-exome sequencing to study the underlying pathogenesis of psoriasis »
Related Articles
September 4, 2019
Efficacy and safety of ixekizumab versus adalimumab in patients with PsA
June 21, 2019
Advances in target-oriented therapy: psoriatic arthritis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

