https://doi.org/10.55788/7741a55a
The phase 3 PROMIS clinical programme (NCT03093974), consisting of the PROMIS-I (n=377) and PROMIS-II (n=287) trials, was designed to evaluate the safety and efficacy of colistimethate sodium powder for nebulisation solution in patients with NCFB who are chronically infected with P. aeruginosa [1]. Dr Charles Haworth (Royal Papworth Hospital, UK) presented the results.
After 12 months, twice-daily administration of nebulised colistimethate sodium powder (n=177) significantly reduced annual pulmonary exacerbation rates compared with placebo (n=200; 0.58 vs 0.95 per patient per year; rate ratio [RR] 0.61; 95% CI 0.46–0.82; P=0.001); thus, meeting the primary endpoint of the trial. The treatment effect was larger in adherent participants (43.5% reduction in exacerbations; P<0.001). The trial also met important secondary endpoints, demonstrating improvements compared with placebo regarding prolonged time to first exacerbation in the nebulised colistimethate sodium powder group (HR 0.59; 95% CI 0.43–0.81; P<0.001, see Figure). The frequency of severe exacerbations was also reduced (RR 0.41; 95% CI 0.23–0.74; P=0.003). Quality of life, measured by the St. George’s Respiratory Questionnaire (SGRQ), significantly improved with colistimethate sodium powder, with a 4.55 point difference versus placebo after 12 months of treatment (P=0.006). After 28 days of treatment, P. aeruginosa density was significantly reduced in the treatment arm (P<0.001). The percentage of patients with adverse events was similar between groups. Bronchospasm and antibiotic resistance were infrequently observed (2.8% and 1%, respectively).
Figure: Time to first exacerbation in PROMIS [1]
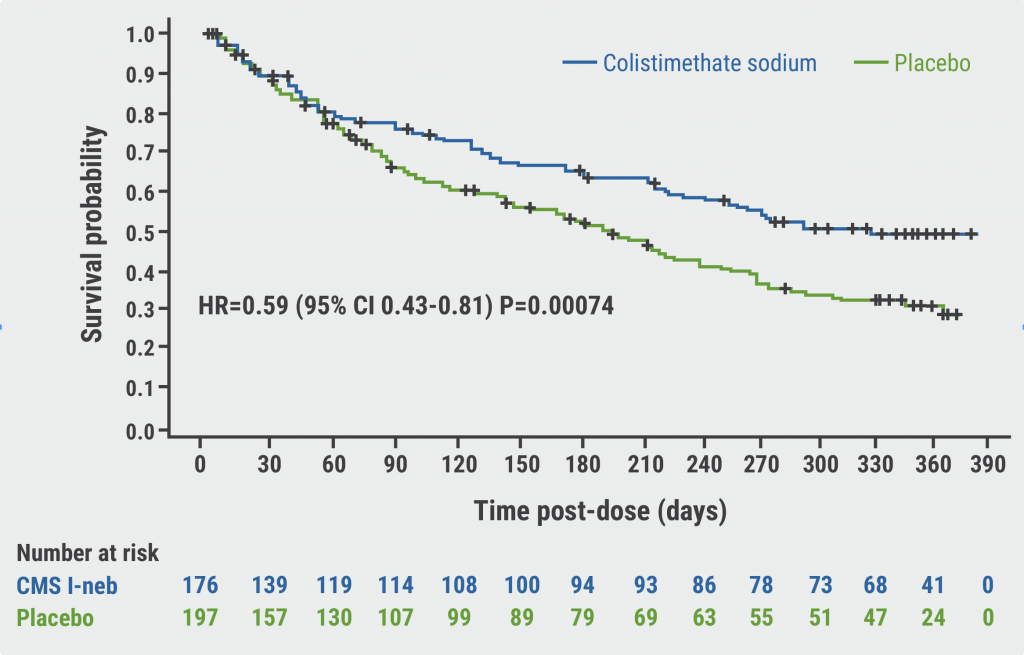
“The PROMIS data shows that colistimethate sodium powder taken twice daily through nebulisation solution reduces exacerbation frequency and improves quality of life in people with bronchiectasis and chronic P. aeruginosa infection,” summarised Dr Haworth. “The data also demonstrates that 12 months of treatment is well tolerated. These results are encouraging for patients as there is currently no approved drug treatment for this indication.”
- Haworth C, et al. Efficacy and Safety of Colistimethate Sodium Delivered Via the I-Neb in Patients with Bronchiectasis and Pseudomonas Aeruginosa. Session B12, ATS International Conference 2022, San Francisco, CA, USA, 13–18 May.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Is avacopan better than prednisone for respiratory ANCA-associated vasculitis outcomes? Next Article
Novel P2X3 antagonist can SOOTHE chronic cough »
« Is avacopan better than prednisone for respiratory ANCA-associated vasculitis outcomes? Next Article
Novel P2X3 antagonist can SOOTHE chronic cough »
Table of Contents: ATS 2022
Featured articles
Letter from the Editor
COVID-19
Nebulised aviptadil “futile” in I-SPY COVID-19 trial
Lung transplantation after COVID-19-associated ARDS
Mesenchymal stem cells offer no benefit in COVID-19
Alpha-1 antitrypsin for ARDS secondary to severe COVID-19
Frailty prevalent 5 months following hospitalisation for COVID-19
Paediatric long COVID lacks definitions
Asthma Clinical Trial Updates
MANDALA and DENALI pattern success for albuterol-budesonide in asthma
ACOUSTICS data sounds good for adolescent asthma exacerbations
Type 2 asthma in children managed by dupilumab, despite atopic comorbidities
NAVIGATOR steers asthma patients to tezepelumab
High-intensity interval training slashes daily corticosteroids in asthma
Chronic Obstructive Pulmonary Disease
Three’s a crowd for triple therapy in COPD
Higher 1-year COPD mortality after hospitalisation for White patients
Reducing dyspnoea in chronic lung disease through weight loss
CT-evident mucus plugs in COPD associated with death
Home-based rehabilitation improves COPD: a randomised study
Highlighted Advances
Novel P2X3 antagonist can SOOTHE chronic cough
Colistimethate sodium PROMISing for non-cystic fibrosis bronchiectasis
Is avacopan better than prednisone for respiratory ANCA-associated vasculitis outcomes?
PAGANINI phase 2b data promising for eliapixant
POISE-3: Tranexamic acid for non-cardiac surgery
Obstructive sleep apnoea in most children with pulmonary hypertension
No screening evidence for COPD
Novel PDE4B inhibitor offers breakthrough for IPF
Hydrocortisone does not help preterm infants
CPAP temporarily supports pulmonary oxygenation in morbidly obese patients
ISAACC trial: CPAP controls blood pressure in ACS patients with severe OSA
Related Articles
August 17, 2022
Severe asthma in the spotlights
October 22, 2020
Extracorporeal CO2 removal effective in status asthmaticus
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

