https://doi.org/10.55788/0336f107
The phase 3, multicentre, randomised, double-blind, placebo-controlled ACOUSTICS study (NCT01875003) aimed to test the efficacy and safety of lebrikizumab, a high-affinity IgG4 monoclonal antibody targeting IL-13, selectively preventing the formation of the IL-13/IL-4 heterodimer receptor signalling complex. The trial was stopped early by the sponsor.
In ACOUSTICS, participants (n=346 adolescents, aged 12 to 17 years) with uncontrolled asthma – despite using inhaled corticosteroids daily in addition to at least 1 other asthma controller medication – were randomised to receive lebrikizumab 125 mg (n=116) or 37.5 mg (n=113), or placebo (n=117) subcutaneously once every 4 weeks. The primary outcome was the asthma exacerbation rate, defined as new or worsened asthma symptoms that led to treatment with systemic corticosteroids of hospital admission. The time to first asthma exacerbation and safety outcomes were also evaluated. Prof. Stanley Szefler (Children’s Hospital Colorado, CO, USA) presented the results from the 224 (65%) adolescents who have completed 52 weeks thus far [1].
Compared with the placebo group, participants assigned lebrikizumab 125 mg had a 51% reduction in exacerbation rates (adjusted RR 0.49; 95% CI 0.28–0.83), and the 37.5 mg arm had a 40% reduction (aRR 0.60; 95% CI 0.35–1.03). Compared with the placebo group, patients in the lebrikizumab arms experienced a longer interval before their first asthma exacerbation for both the 37.5 mg dose (HR 0.40; 95% CI 0.22–0.73) and the 125 mg dose (HR 0.37; 95% CI 0.21–0.66).
The baseline median blood eosinophil count was 295 cells/µL; the researchers accordingly looked at the data using a threshold baseline blood eosinophil count of ≥300 cells/µL. In those with blood eosinophil counts of ≥300 cells/µL, the lebrikizumab 125 mg arm had a reduction of 56% (RR 0.44; 95% CI 0.21–0.89) in asthma exacerbation rates, but that rate was similar in the 37.5 mg arm with a 58% reduction (RR 0.42; 95% CI 0.19–0.93) (see Figure).
Figure: Reduction in asthma exacerbation rates with lebrikizumab versus placebo was greatest in patients with eosinophils ≥300 cells/µL [1]
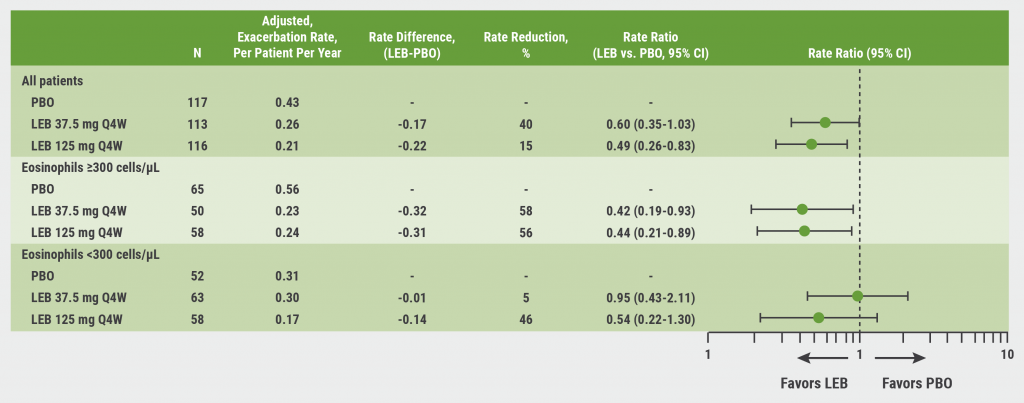
CI, confidence interval; LEB, lebrikizumab; PBO, placebo; Q4W, every 4 weeks.
Most adverse events that occurred during the study were mild-to-moderate in severity and did not lead to discontinuation of the study drug. Eosinophil-associated, treatment-related adverse events included decreased neutrophil count and eosinophilia; there were no cases of eosinophilic granulomatosis with polyangiitis. “In terms of safety, it was pretty comparable to what was seen in the adult studies to date,” Prof. Szefler said.
Prof. Szefler concluded that there was a greater effect observed with the higher dose in the overall population and that exacerbation rates were trending toward further reduction in patients with baseline eosinophilia. He pointed out that, despite the lack of a consistent dose-response, post-hoc analyses of adult studies (e.g. LAVOLTA I and II, MILLY) showed similar results. This data collectively supports additional research into the optimal use of lebrikizumab with higher and more frequent dosing in patients with type 2 inflammation at risk for exacerbations.
- Szefler SJ, et al. Efficacy, Safety, and Tolerability of Lebrikizumab in Adolescent Patients with Uncontrolled Asthma (ACOUSTICS): A Phase 3, Randomised, Double-Blind, Placebo-Controlled Study. Session B93, ATS International Conference 2022, San Francisco, CA, USA, 13–18 May.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Type 2 asthma in children managed by dupilumab, despite atopic comorbidities Next Article
MANDALA and DENALI pattern success for albuterol-budesonide in asthma »
« Type 2 asthma in children managed by dupilumab, despite atopic comorbidities Next Article
MANDALA and DENALI pattern success for albuterol-budesonide in asthma »
Table of Contents: ATS 2022
Featured articles
Letter from the Editor
COVID-19
Nebulised aviptadil “futile” in I-SPY COVID-19 trial
Lung transplantation after COVID-19-associated ARDS
Mesenchymal stem cells offer no benefit in COVID-19
Alpha-1 antitrypsin for ARDS secondary to severe COVID-19
Frailty prevalent 5 months following hospitalisation for COVID-19
Paediatric long COVID lacks definitions
Asthma Clinical Trial Updates
MANDALA and DENALI pattern success for albuterol-budesonide in asthma
ACOUSTICS data sounds good for adolescent asthma exacerbations
Type 2 asthma in children managed by dupilumab, despite atopic comorbidities
NAVIGATOR steers asthma patients to tezepelumab
High-intensity interval training slashes daily corticosteroids in asthma
Chronic Obstructive Pulmonary Disease
Three’s a crowd for triple therapy in COPD
Higher 1-year COPD mortality after hospitalisation for White patients
Reducing dyspnoea in chronic lung disease through weight loss
CT-evident mucus plugs in COPD associated with death
Home-based rehabilitation improves COPD: a randomised study
Highlighted Advances
Novel P2X3 antagonist can SOOTHE chronic cough
Colistimethate sodium PROMISing for non-cystic fibrosis bronchiectasis
Is avacopan better than prednisone for respiratory ANCA-associated vasculitis outcomes?
PAGANINI phase 2b data promising for eliapixant
POISE-3: Tranexamic acid for non-cardiac surgery
Obstructive sleep apnoea in most children with pulmonary hypertension
No screening evidence for COPD
Novel PDE4B inhibitor offers breakthrough for IPF
Hydrocortisone does not help preterm infants
CPAP temporarily supports pulmonary oxygenation in morbidly obese patients
ISAACC trial: CPAP controls blood pressure in ACS patients with severe OSA
Related Articles
October 30, 2022
Does vilobelimab reduce mortality in severe COVID-19?

May 24, 2017
Letter from the Editor
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

