https://doi.org/10.55788/5b812b5a
CDK4/6 inhibitors have been shown to improve the outcome of patients with advanced/metastatic ER+/HER2- breast cancer in the first and second line [1,2]. Most guidelines advise using it as a first-line treatment despite a lack of comparative evidence. However, first-line use is associated with prolonged side effects and higher drug costs [3].
The (publicly funded) Dutch, nationwide, randomised, phase 3 SONIA trial (NCT03425838) compared the use of CDK4/6 inhibitors as first- or second-line treatment in patients with advanced/metastatic ER+/HER2- breast cancer. Prof. Gabe Sonke (Netherlands Cancer Institute, the Netherlands) presented the results [4].
In the study, 1,050 participants were 1:1 randomised to first-line non-steroidal aromatase inhibitor plus a CDK4/6 inhibitor followed by second-line fulvestrant (first-line arm) or to first-line non-steroidal aromatase inhibitor followed by second-line fulvestrant plus a CDK4/6 inhibitor (second-line arm). The primary endpoint was progression-free survival after 2 lines of treatment (PFS2).
Although first-line CDK4/6 inhibition favoured PFS after 1 line of therapy, PFS2 was not statistically different between arms: 31.0 versus 26.8 months (HR 0.87; P=0.10) in the first-line arm and second-line arm, respectively (see Figure). In addition, overall survival was not significantly different between arms: 45.9 versus 53.7 months (HR 0.98; P=0.83).
Figure: PFS after 2 lines of treatment in the SONIA trial [4]
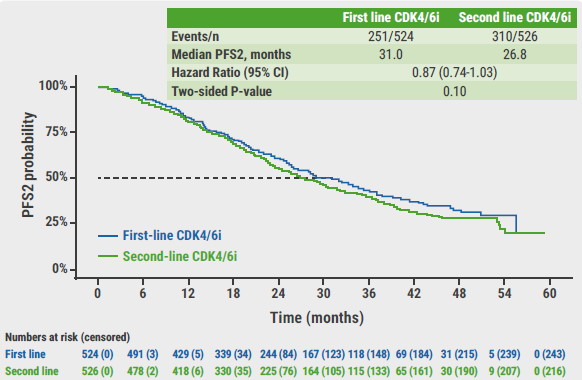
CI, confidence interval; PFS, progression-free survival
First-line CDK4/6 inhibition came with 42% more grade ≥3 adverse events compared with second-line CDK4/6 inhibition (n=2,782 vs n=1,620), most likely due to an extended time on CDK4/6 inhibition. For the same reason, first-line CDK4/6 inhibition also increased drug expenditure by $200,000 per patient.
Based on these results, Prof. Sonke concluded that “CDK4/6 inhibition in first line compared with second line does not improve survival, does not improve quality-of-life, increases toxicity, and increases costs. Therefore, second-line CDK4/6 inhibition should be standard-of-care until we know which patients benefit from first-line CDK4/6 inhibition over second-line CDK4/6 inhibition.”
- Goetz MP, et al J Clin Oncol. 2017;35:3638–3646.
- Sledge G, et al. J Clin Oncol 2017;35:2875–2884.
- Van Ommen-Nijhof A, et al. BMC Cancer. 2018;18(1):1146.
- Sonke GS, et al. Primary outcome of the phase 3 SONIA trial (BOOG 2017-03). Abstract LBA1000, ASCO Annual Meeting 2023, 2–6 June, Chicago, USA.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Adjuvant ribociclib improves invasive DFS in early breast cancer Next Article
De-escalation of neoadjuvant treatment of locally advanced rectal cancer is non-inferior »
« Adjuvant ribociclib improves invasive DFS in early breast cancer Next Article
De-escalation of neoadjuvant treatment of locally advanced rectal cancer is non-inferior »
Table of Contents: ASCO 2023
Featured articles
Real-world data support new SOC in patients with SCLC
What can real-world evidence teach us about atezolizumab plus bevacizumab in HCC?
Colorectal Cancer
7-year outcomes of PRODIGE 23 trial
Neoadjuvant chemotherapy may be viable option in locally advanced colon cancer
De-escalation of neoadjuvant treatment of locally advanced rectal cancer is non-inferior
Breast Cancer
SONIA: No survival benefit with first-line versus second-line CDK4/6 inhibition in metastatic breast cancer
Adjuvant ribociclib improves invasive DFS in early breast cancer
Gene expression profiles predict benefit of neoadjuvant immune checkpoint therapy in triple-negative breast cancer
Lung Cancer
Adding pembrolizumab to perioperative chemotherapy improves EFS in early-stage NSCLC
TTFields therapy: a new treatment modality for metastatic NSCLC
Adding chemotherapy to EGFR TKI does not improve OS in advanced EGFR-mutated NSCLC
Upper GI Cancer
No improved OS in pancreatic cancer after neoadjuvant mFOLFIRINOX
AI detects gastric cancer with high accuracy in common blood tests
Melanoma
Response-directed treatment personalisation in stage III melanoma
Prognostic and predictive biomarkers in patients with resected stage IIB/C melanoma
GU Cancers
Combining PARP inhibition and androgen receptor-signalling inhibition improves radiographic progression-free survival in HRR-deficient mCRPC
Erdafitinib outperforms chemotherapy in FGFR-altered advanced urothelial cancer
Probiotic CBM588 seems to improve clinical effect cabozantinib/nivolumab in mRCC
Exploratory analysis of IMvigor130 trial finds no OS benefit from atezolizumab in subgroups
Miscellaneous
Immune checkpoint inhibition improves PFS in non-BRCA-mutated ovarian cancer
First-line nivolumab-AVD improves PFS both in adult and paediatric patients with advanced Hodgkin lymphoma
Vorasidenib successfully targets IDH1/2-mutated glioma
ASCO Interviews
IMbrave050: Adjuvant atezolizumab plus bevacizumab provides landmark recurrence-free survival for HCC
What can real-world evidence teach us about atezolizumab plus bevacizumab in HCC?
Related Articles
November 19, 2021
High COVID-19 mortality in Swiss cancer patients
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

