Compared with placebo, which was tied to a 26.5% reduction in seizure rate, an oral dose of 25 mg/kg/day of cannabidiol lowered the rate by 48.6% and a dose twice as large produced a 47.5% reduction, but with more side effects.
In fact, "half of the patients randomized to 50 mg/kg/day (were) unable to reach or maintain that dosage" because of side effects, the researchers said.
"I think CBD is a very important addition to our treatment options for patients with TSC, as 85% of individuals develop epilepsy, which is refractory to medication in about two thirds (compared to one third in the general epilepsy population)," chief author Dr. Elizabeth Thiele of Massachusetts General Hospital in Boston told Reuters Health in an email.
The drug is already approved as a treatment for seizures in patients with Dravet and Lennox-Gastaut syndromes. The U.S. Food and Drug Administration approved CBD for seizures associated with TSC in patients 1 year and older this summer.
The findings are published in JAMA Neurology. GW Pharmaceuticals, which sells the drug under the brand name Epidiolex in the U.S. and Epidyolex in the EU, paid for the study.
TSC is a rare genetic disease that affects an estimated 1 to 2 million people worldwide and is marked by benign potato-like tumors in the brain and other organs. Seizures are common, but don't always manifest as convulsions. They may alter awareness or behavior.
In patients who experience seizures, more than 60% have treatment-resistant epilepsy.
The study, known as GWPCARE6, included 224 volunteers from six countries who were spread almost evenly between the three groups. All the patients had experienced at least eight seizures during a four-week baseline period.
"Patients in this study had frequent and highly treatment-resistant seizures; cannabidiol was their eighth attempted medication on average," the researchers wrote.
Study participants received cannabidiol or placebo for 16 weeks followed by a 10-day tapering period and four additional weeks of monitoring for safety. All the patients continued to get conventional therapy.
A 50% reduction in seizures was seen in 36% of the volunteers taking the lower dose of the drug, 40% getting the higher dose and 22% of placebo recipients. Only the difference between the higher dose and placebo was statistically significant.
Nearly 17% of cannabidiol recipients had a reduction in their seizure rate of at least 75%. None of the volunteers in the placebo group saw that degree of reduction.
"Cannabidiol led to meaningful reductions in seizures vs placebo observed as early as the titration period and maintained throughout the study," the researchers write.
When the patients themselves were asked if their condition had improved, 69% in the low-dose group, 62% in the high-dose group and 39% in the placebo group said it had.
The most common side effect was diarrhea, seen in 56% of the patients getting the 50-mg dose, 31% of those getting 25 mg/kg/day and 25% for placebo recipients. Most cases were mild.
Excessive sleepiness was seen in 26%, 13% and 9% respectively, with only the higher dose producing a problem rated as moderate or severe.
The drug also increased liver transaminase levels in 19% of recipients.
An adverse event prompted discontinuation of treatment in 11% of patients in the low-dose group, 14% in the high-dose group and 3% in the placebo group.
Based on the new findings, "I do think that many physicians will try to prescribe it off label, as there are many individuals with treatment refractory epilepsy from many different etiologies," said Dr. Thiele, director of the pediatric epilepsy program at Mass General. "There remains a significant unmet need for effective, safe and well tolerated antiseizure medications, and I think that CBD is a good addition to our treatment options."
Data on long-term effectiveness have not been published, but Dr. Thiele said "data from the open label extension of the trial were recently presented at the American Epilepsy Society meeting, showing continued efficacy and tolerability."
SOURCE: https://bit.ly/2WyqmwR JAMA Neurology, online December 21, 2020.
By Gene Emery
Posted on
Previous Article
« Assessing measurable residual disease tied to better outcome in multiple myeloma Next Article
Delaying cancer treatment during pandemic likely unnecessary »
« Assessing measurable residual disease tied to better outcome in multiple myeloma Next Article
Delaying cancer treatment during pandemic likely unnecessary »
Related Articles
November 8, 2019
Late-breaking: Myelin-peptide coupled red blood cells
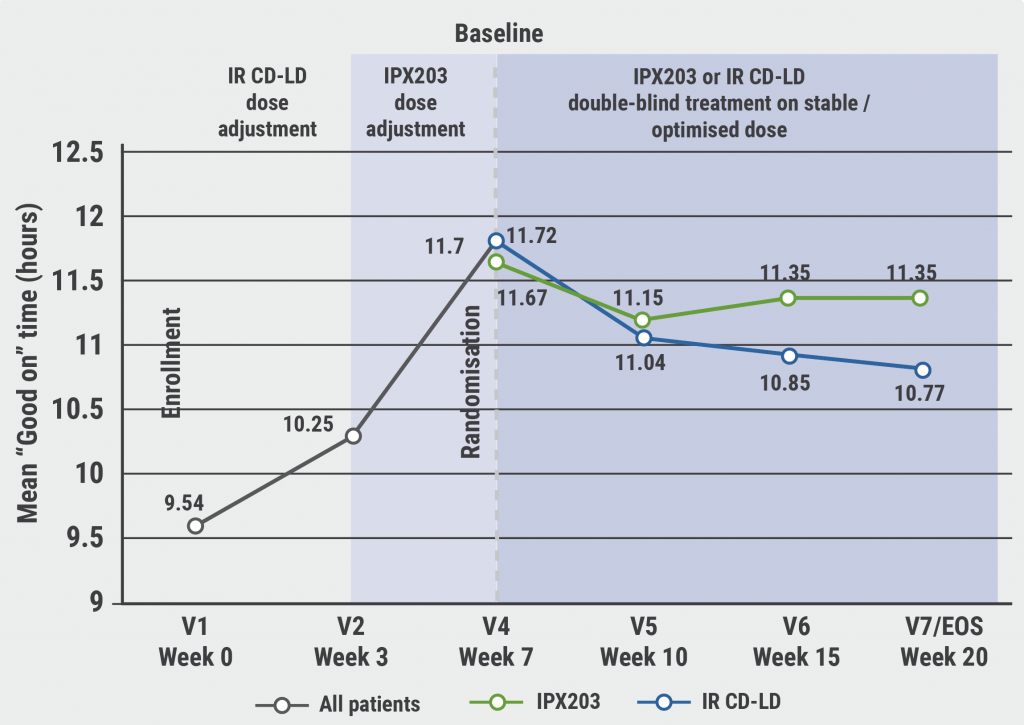
June 2, 2022
IPX203 versus immediate release carbidopa-levodopa
August 18, 2021
Stroke with covert brain infarction indicates high vascular risk
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

