https://doi.org/10.55788/c441edbd
IPX203 was specifically designed to provide rapid levodopa absorption to quickly reach the desired plasma concentration and to maintain levodopa concentrations within the therapeutic range for a longer period of time than immediate release CD-LD, and with less peak-to-trough fluctuation. Prof. Robert Hauser (USF Parkinson's Disease and Movement Disorders Center, FL, USA) explained that the phase 3 RISE-PD study (NCT03670953) was meant to test the efficacy and safety of IPX203 and enrolled 506 participants aged 40 years and older (mean age of 66 years) with PD and motor fluctuations, who had at least 2.5 hours daily “off” time (no adequate symptom control) on average during waking hours [1].
For the first 3 weeks, participants underwent dose-adjusting, open-label treatment with immediate release CD-LD, followed by 4 weeks of open-label IPX203 treatment. Participants then entered a double-blind maintenance phase of 13 weeks, where they received either IPX203 (n=256), dosed on average 3 times a day (never more frequently than every 6 hours), or standard immediate-release CD-LD (n=250), dosed on average 5 times a day. Dosing adjustments were allowed to achieve an optimal response. Efficacy was measured at week 20 and the primary endpoint was “good on” time in hours per day, defined as the sum of "on" time without dyskinesia and “on” time with non-troublesome dyskinesia.
The results showed both an improvement from baseline in terms of “good on” time and a corresponding reduction in “off” time (see Figure). IPX203 led to an average of 0.53 more hours of “good on” time per day versus immediate-release CD-LD (P=0.0194). Consistently, “off” time was reduced by 0.48 hours per day on average (P=0.0252). The Patient Global Impression of Change (PGI-C) score, based on clinician assessment, showed that 29.7% of participants treated with IPX203 had a much or very much improved general health, versus 18.8% of participants treated with immediate-release CD-LD (P=0.0015). Change from baseline in the assessment of disability (Movement Disorders Society–Unified Parkinson’s disease Rating Scale [MDS-UPDRS] Part III scores) was similar in both treatment groups. Prof. Hauser stressed the “critical importance” of the observation that the significant differences in favour of IPX203 were apparent despite it being dosed on average only 3 times a day. The most common treatment-emergent adverse effects were nausea, falls, and urinary tract infections.
Figure: IPX203 demonstrated significant improvement “good on” time from baseline to end of study [1]
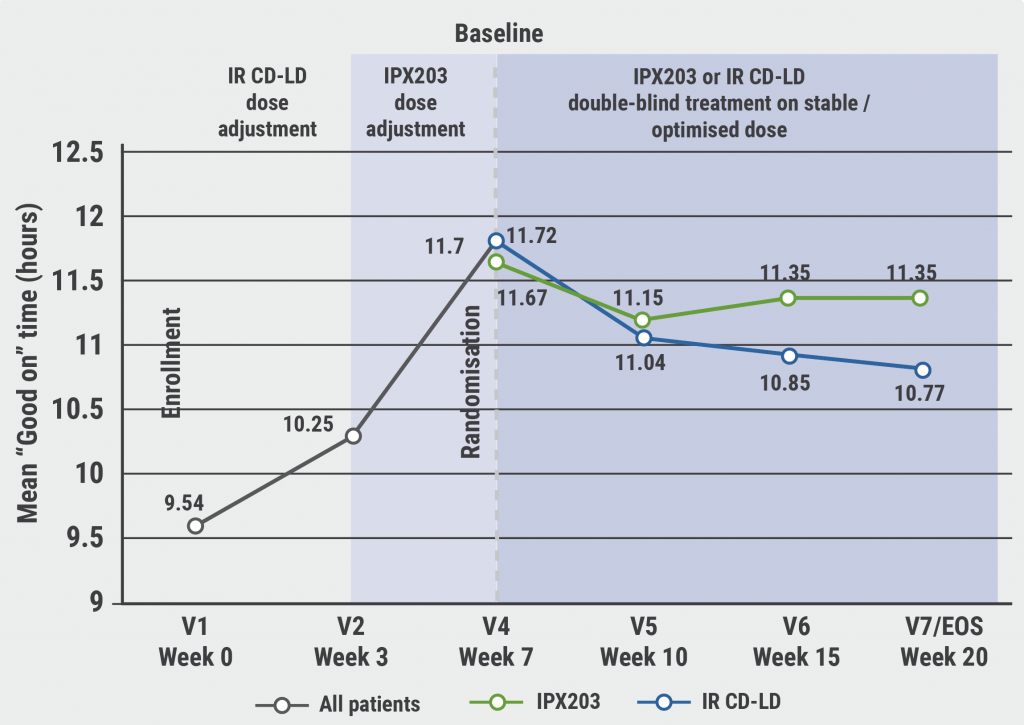
IR CD-LD, immediate release carbidopa-levodopa.
A poster reported the mean duration of “good on” time per dose for IPX203 versus immediate-release CD-LD, which was established in a post-hoc analysis [2]. IPX203 provided 1.55 more hours (3.76 vs 2.21 hours) of “good on” time per dose, representing a 70% increase.
- Hauser RA, et al. A phase 3 trial of IPX203 vs CD-LD IR in Parkinson’s Disease patients with motor fluctuations (RISE-PD). S16.010, AAN 2022, 02–07 April, Seattle, USA.
- Hauser RA, et al. Duration of benefit per dose: Post hoc analysis of “good on” time per dose for IPX203 vs CD-LD IR in the RISE-PD Phase 3 trial. P10.002, AAN 2022, 02–07 April, Seattle, USA.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Impact of COVID-19 public health interventions Next Article
Prasinezumab in Parkinson’s disease: delayed-start analysis of PASADENA trial »
« Impact of COVID-19 public health interventions Next Article
Prasinezumab in Parkinson’s disease: delayed-start analysis of PASADENA trial »
Table of Contents: AAN 2022
Featured articles
Letter from the Editor
Interview with Prof. Natalia Rost
Alzheimer’s Disease and Other Dementias
Targeting senescent cells to treat age-related diseases
Cardiorespiratory fitness protects against dementia
Safety and effects of bosutinib in Lewy body dementia
Epilepsy
“Women with epilepsy should be encouraged to breastfeed”
Fenfluramine: possible new treatment for Lennox-Gastaut syndrome
Laser interstitial thermal therapy for refractory epilepsy
Migraine
Migraine may be an important obstetric risk factor
Intranasal zavegepant safe and well tolerated in healthy adults
Telemedicine during COVID-19 pandemic highly appreciated
Multiple Sclerosis
Ublituximab versus teriflunomide in relapsing MS patients
Ketogenic diet may improve disability and quality of life
Favourable additional safety data for ofatumumab
Predicting new T2 lesions using a machine learning algorithm
Evobrutinib reduces volume of slowly expanding lesions
Sustained long-term efficacy and safety of satralizumab in NMOSD
Muscle and Neuro-Muscular Disorders
Ravulizumab in patients with generalised myasthenia gravis
Gene therapy effective in older patients with spinal muscular atrophy
Losmapimod for facioscapulohumeral muscular dystrophy
SRP-9001 for treating patients with Duchenne muscular dystrophy
Cerebrovascular Disease and Stroke
Intravenous thrombolysis after ischaemic stroke: When in doubt, leave it out?
Better outcomes with mechanical thrombectomy in elderly stroke patients
Plasma NfL levels associated with cardiovascular risk
Non-invasive vagus nerve stimulation for acute stroke
Parkinson’s Disease
Prasinezumab in Parkinson’s disease: delayed-start analysis of PASADENA trial
IPX203 versus immediate release carbidopa-levodopa
Impact of COVID-19 public health interventions
COVID-19
Cognitive, EEG, and MRI features in COVID-19 survivors
Neurological manifestations of COVID-19 worsen prognosis
New evidence for biological basis of “COVID-19 brain fog”
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

