After a successful trial with myelin peptide-coupled peripheral blood mononuclear cells (PBMCs) in MS patients, the researchers optimised the approach using red blood cells (RBCs) as tolerogenic carriers (see Figure) [2]. ETIMSred is the first-in-human phase 1b trial, to test the safety and tolerability of increasing doses of autologous peptide-coupled RBCs. Ten relapsing-remitting MS patients (mean age 38.5; 70% female) were treated with increasing doses of peptide-coupled RBCs.
Figure. Tolerance induction with peptide-coupled RBCs or white blood cells [2]
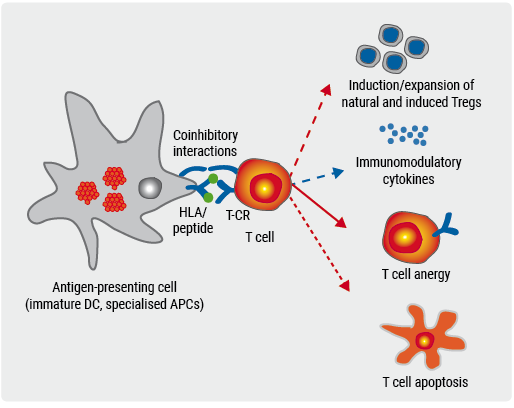
The trial met its primary endpoint, demonstrating feasibility, safety, and excellent tolerability. There were no adverse events within the 24 hours after infusion and no serious adverse events occurred in the trial. Patients remained stable in all clinical parameters. The trial was accompanied by mechanistic studies to assess in vivo immunological effects of the therapy [2]. Frequency of myelin peptide-specific T cells reduced after tolerization (more pronounced in high-dose patients), which suggests there was antigen-specific tolerization. Increases in regulatory T cells and IL-10, as well as a reduction of neurofilament light chain, point towards active mechanisms of immune tolerance [2]. The next step is to expand the peptide cocktail to novel antigens. A phase 2a trial to assess the efficacy of the approach is being developed.
- Turley DM, Miller SD. J Immunol. 2007;178:2212-20.
- Lutterotti A, et al. ECTRIMS 2019, abstract 339.
Posted on
Previous Article
« Targeted therapies for NMOSD in development Next Article
Late-breaking: Ofatumumab versus teriflunomide in relapsing MS »
« Targeted therapies for NMOSD in development Next Article
Late-breaking: Ofatumumab versus teriflunomide in relapsing MS »
Table of Contents: ECTRIMS 2019
Featured articles
Towards a Comprehensive Assessment of MS Course
Cognitive assessment in MS
Late-breaking: Role for CSF markers in autoimmune astrocytopathies
Targeted therapies for NMOSD in development
Monitoring and Treatment of Progressive MS
Challenges in diagnosing and treating progressive MS
Risk factors for conversion to secondary progressive MS
Transplantation of autologous mesenchymal stem cells
Sustained reduction in disability progression with ocrelizumab
Late-breaking: Myelin-peptide coupled red blood cells
Optimising Long-Term Benefit of MS Treatment
Induction therapy over treatment escalation
Treatment escalation over induction therapy
Influence of age on disease progression
Exposure to DMTs reduces disability progression
Predicting long-term sustained disability progression
Treatment response scoring systems to assess long term prognosis
Safety Assessment in the Post-Approval Phase
Use of clinical registries in phase 4 of DMT
Genes, environment, and safety monitoring in using registries
Risk of hypogammaglobulinemia and rituximab
Determinants of outcomes for natalizumab-associated PML
Serum immunoglobulin levels and risk of serious infections
EAN guideline on palliative care
Pregnancy in the Treatment Era
The maternal perspective: when to stop/resume treatment and risks for progression
Foetal/child perspective: risks related to drug exposure and breastfeeding
Patient awareness about family planning represents a major knowledge gap
Late-breaking: Continuation of natalizumab or interruption during pregnancy
Related Articles
November 8, 2019
Determinants of outcomes for natalizumab-associated PML
November 8, 2019
Risk of hypogammaglobulinemia and rituximab
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

