https://doi.org/10.55788/134db293
An immune response to COVID-19 vaccines varies between different DMTs used for MS. How does this translate into preventing COVID-19 and severe outcomes of the infection among MS patients? In order to assess this, a prospective and longitudinal cohort was formed of all MS patients in Great Britain who were using any DMT between March 2020 and August 2022. Data from 36,779 MS patients was provided by the National Health Service England and United Kingdom Health and Security Agency and comprised all COVID-19 tests, outcomes, and vaccinations. Outcomes were COVID-19 incidence, hospitalisation, and mortality compared with the general population and COVID-19-related hospitalisation and mortality in vaccinated patients. The results were presented by Dr Nikos Evangelou (Nottingham University Hospital, UK) [1,2].
Some of the results showed that in 2021, 80% of MS hospitalisations which involved COVID-19 had COVID-19 as primary diagnosis. In 2022 (until the end of August), this percentage went down to 50%. The most frequently used DMT among hospitalised MS patients with COVID-19 as primary diagnosis was ocrelizumab: 2.3% in 2021 and almost 1.5% in 2022. Looking only at MS patients who had received 2 COVID-19 vaccinations, rate of hospitalisation with COVID-19 as primary diagnosis was highest among users of anti-CD20 medications: 13 per 100 for ocrelizumab and 7.9 per 100 for fingolimod. COVID-19-related in-hospital mortality (per 1,000 people) was 0.3 for fingolimod, 2 for ocrelizumab, and 0–1.3 for other DMTs. Following a third vaccination in 2021 in the UK, no hospitalisations were measured with any of the MS DMTs except for fingolimod (14%) and ocrelizumab (6%); none of these patients died.
Generally speaking, in 2022, mortality decreased possibly due to the widespread use of antivirals and the relatively milder Omicron variant. On the other hand, vaccination rates were declining. How to explain to an MS patient the implications on a group level of being or not being vaccinated? The presented graph reflects the situation in the UK, according to Dr Evangelou (see Figure).
Figure: Effect of COVID-19 infection on MS patients in the UK on a DMT [1]
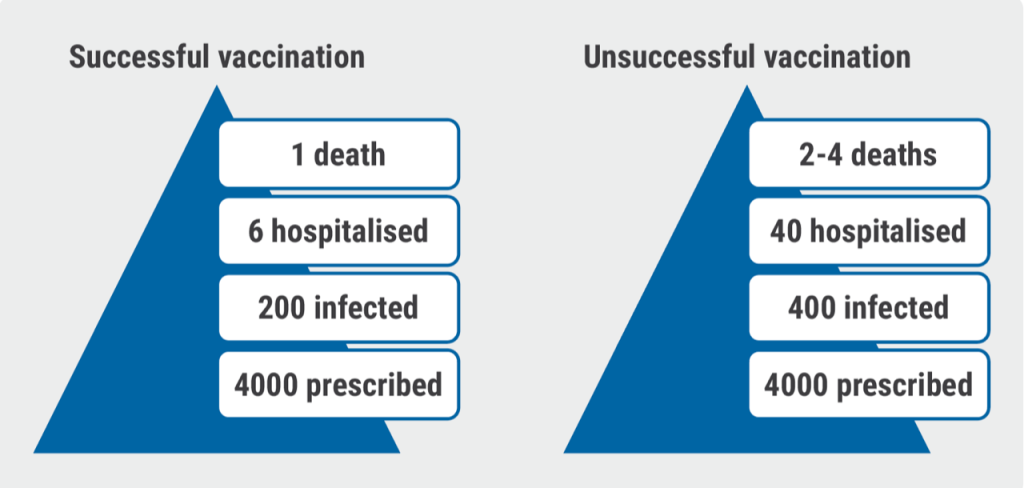
- Evangelou N. Effectiveness of COVID-19 vaccines in people with multiple sclerosis receiving disease-modifying therapies: a total population study of National Health Service (NHS) England. Abstract O137, ECTRIMS 2022, 26–28 October, Amsterdam, the Netherlands.
- Garjani A, et al. Mult Scler Relat Disord. 2022;57:103458.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Humoral and cellular immune responses after SARS-CoV-2 vaccination Next Article
COVID-19 and MS: lessons learned thus far »
« Humoral and cellular immune responses after SARS-CoV-2 vaccination Next Article
COVID-19 and MS: lessons learned thus far »
Table of Contents: ECTRIMS 2022
Featured articles
Letter from the Editor
Diagnosis and Prediction of Disease Course
A case for including optic nerve lesions in the McDonald criteria
Cerebrospinal fluid kappa-free light chains for MS diagnosis
Early, non-disabling relapses increase disability accumulation
Physical impairment is present before perceived MS onset
Chronic active MS lesions respond poorly to anti-CD20 antibodies
Treatment: Trials & Strategies
Dimethyl fumarate reduces the risk of a first clinical event in RIS
How and when to make a timely switch to high-efficacy DMT
Comparing real-world effectiveness of DMTs
Study fails to show non-inferiority of rituximab to ocrelizumab
Autologous haematopoietic stem cell transplantation versus DMTs
Progressive MS
Stem cell transplantation not superior to natalizumab in progressive MS
Efficacy of DMTs fades away in secondary progressive MS
Smartphone tapping can help detect progressive MS
Paediatric MS
Early treatment with DMT effective in paediatric-onset MS
Fingolimod in paediatric MS: results of up to 6 years
Switching treatment after initial platform injectable DMT: real-world data
Pregnancy
Pregnancy and infant outcomes in women receiving ocrelizumab
New safety data of anti-CD20 mAbs around pregnancy
MS activity and pregnancy outcomes after long-term use of natalizumab
NMOSD
Ravulizumab significantly reduced relapses in AQP4+ NMOSD
NMOSD patients are cognitively impaired regardless of serostatus
Evidence-based consensus on pregnancy in NMOSD
COVID-19
COVID-19 and MS: lessons learned thus far
Ocrelizumab and fingolimod increase the risk of COVID-19 and of worse outcomes
Humoral and cellular immune responses after SARS-CoV-2 vaccination
Miscellaneous
Re-myelination strategies in MS still pose many unanswered questions
MS associated with a broader Epstein-Barr virus specific T-cell receptor repertoire
Cognitive rehab and mindfulness reduce cognitive complaints in MS
Related Articles
December 20, 2022
Autologous haematopoietic stem cell transplantation versus DMTs
November 18, 2024
Revised McDonald criteria allow earlier and more precise MS diagnosis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

