To determine when to stop and when to resume MS treatment, it is important to consider the disease course during and after pregnancy. The European multicentric Pregnancy in Multiple Sclerosis (PRIMS) study, which was conducted in the period before the introduction of disease-modifying drugs (DMTs), clearly showed that disease activity reduced during the gestational period (P=0.03) and increased thereafter, particularly during the first trimester postpartum [1].
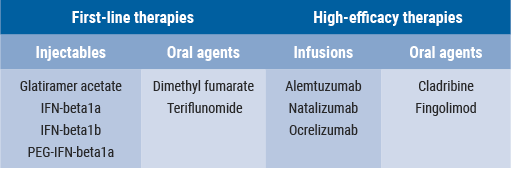
Since the publication of the PRIMS study, several DMTs have been introduced (Table). “With respect to disease course and maternal risk of progression during pregnancy, that risk is generally limited for the newer drugs”, Dr Portaccio said. “These drugs may be valuable as first-line therapies, particularly the injectables, and among the high-efficacy therapies, natalizumab.” Several studies showed that disease activity during and after pregnancy was reduced in patients receiving first-line therapies, in comparison with the observations in the previous PRIMS study. Therefore, early DMT resumption should be encouraged, particularly in patients with a more active disease [3].
Table. DMTs for the treatment of relapsing-remitting MS [2]
Postpartum period
The postpartum period is a critical phase in which disease reactivation is expected. “Treatment should be aimed to prevent disease reactivation”, Dr Portaccio advised. “We should take into account possible predictors of disease reactivation after delivery. The occurrence of relapses during pregnancy is found to be associated with a higher risk of disease reactivation after delivery. Interestingly, in the Italian pregnancy dataset [3], early restart of disease therapy after delivery reduced the risk of relapses after pregnancy.” However, it was also stated that disease activity after pregnancy is not limited to relapses. “Although the median disease activity (EDSS score) remains stable before and after pregnancy, at the individual level, 13% of patients were observed to have progression of disability after 1-year follow-up [3]. This progression was related to higher disease activity before pregnancy and the occurrence of relapses during the year before delivery.” However, published literature to-date reveals that more studies report no effect of pregnancy on MS disability than those reporting reduced disability or a delay in progression. One of the major limitations is the possibility of reverse causality, i.e. women with more aggressive disease may choose not to conceive due to concerns about their current symptoms, disability, or choice of treatment during pregnancy [4].
In conclusion, pregnancy should be planned after a period of clinical stability, i.e. no relapses in the year before conception. “Next, we should consider risk of postpartum disease activity: relapses before pregnancy, relapses during pregnancy, and higher disability level. Particularly in high risk women, continuation of therapy until conception or during gestational period and early restart of therapy after delivery are recommended.”
- Confavreux C, et al. N Engl J Med. 1998;339:285-91.
- Rotstein D, Montalban X. Nat Rev Neurol. 2019;15:287-300.
- Portaccio E, et al. J Neurol Neurosurg Psychiatry. 2014;85:845-50.
- Nguyen AL, et al. Autoimmun Rev. 2019;18:102360.
Posted on
Previous Article
« Challenges in diagnosing and treating progressive MS Next Article
Letter from the Editor »
« Challenges in diagnosing and treating progressive MS Next Article
Letter from the Editor »
Table of Contents: ECTRIMS 2019
Featured articles
Towards a Comprehensive Assessment of MS Course
Cognitive assessment in MS
Late-breaking: Role for CSF markers in autoimmune astrocytopathies
Targeted therapies for NMOSD in development
Monitoring and Treatment of Progressive MS
Challenges in diagnosing and treating progressive MS
Risk factors for conversion to secondary progressive MS
Transplantation of autologous mesenchymal stem cells
Sustained reduction in disability progression with ocrelizumab
Late-breaking: Myelin-peptide coupled red blood cells
Optimising Long-Term Benefit of MS Treatment
Induction therapy over treatment escalation
Treatment escalation over induction therapy
Influence of age on disease progression
Exposure to DMTs reduces disability progression
Predicting long-term sustained disability progression
Treatment response scoring systems to assess long term prognosis
Safety Assessment in the Post-Approval Phase
Use of clinical registries in phase 4 of DMT
Genes, environment, and safety monitoring in using registries
Risk of hypogammaglobulinemia and rituximab
Determinants of outcomes for natalizumab-associated PML
Serum immunoglobulin levels and risk of serious infections
EAN guideline on palliative care
Pregnancy in the Treatment Era
The maternal perspective: when to stop/resume treatment and risks for progression
Foetal/child perspective: risks related to drug exposure and breastfeeding
Patient awareness about family planning represents a major knowledge gap
Late-breaking: Continuation of natalizumab or interruption during pregnancy
Related Articles
December 9, 2021
MS patients at risk of hampered immune response after vaccination
November 18, 2024
Risk factors and importance of persistent PIRA
August 18, 2021
Typing behaviour to remotely monitor clinical MS status
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

