Two phase 3 trials, identical in design and conducted in parallel, ASCLEPIOS I and II (n=1,881 totally), evaluated the effect of ofatumumab in patients with relapsing MS. Ofatumumab demonstrated significant reduction of adjusted annualised relapse rate (ARR):
- ASCLEPIOS I: 0.22 in teriflunomide group vs 0.11 in ofatumumab group (relative reduction of 50.5%; P<0.001); and
- ASCLEPIOS II: 0.25 in teriflunomide group vs 0.10 in ofatumumab group (relative reduction of 58.5%; P<0.001, see Figure) [2].
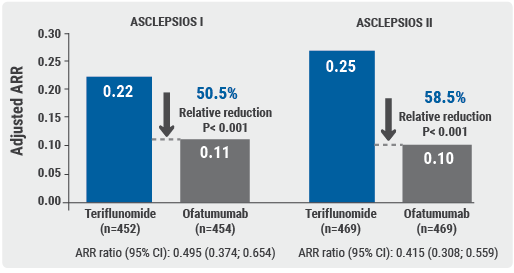
Furthermore, ofatumumab showed a significant reduction in 3- and 6-month confirmed disability worsening (CDW) and a favourable, but non-significant trend to achieve 6-month confirmed disability improvement (CDI):
- 3-month CDW: 15.0% in teriflunomide group vs 10.9% in ofatumumab group (relative reduction of 34.4%; P=0.002; HR 0.656);
- 6-month CDW: 12.0% in teriflunomide group vs 8.1% in ofatumumab group (relative reduction of 32.5%; P=0.012; HR 0.675); and
- 6-month CDI: 8.1% in teriflunomide group vs 11.0% in ofatumumab group (relative increase of 35.2%; P=0.094; HR 1.352) [2].
The ASCLEPIOS I and II trials demonstrated, in a broad population of patients with active, somewhat advanced relapsing MS, that ofatumumab has superior efficacy compared with teriflunomide in lowering relapse rates and MRI activity. Treatment with ofatumumab leads to substantial and significant reductions in 3- and 6-month CDW, and to lower levels of neurofilament light chain (NfL) already at month 3 and at all subsequent visits. Ofatumumab also had a favourable safety profile with no unexpected safety signals. There was no imbalance in the rates of infections or malignancies (low on both arms of the trial).
- Dalakas MC. Nat Clin Pract Neurol. 2008;4:557-67.
- Hauser SL, et al. ECTRIMS 2019, abstract 336.
Posted on
Previous Article
« Late-breaking: Myelin-peptide coupled red blood cells Next Article
Letter from the Editor »
« Late-breaking: Myelin-peptide coupled red blood cells Next Article
Letter from the Editor »
Table of Contents: ECTRIMS 2019
Featured articles
Towards a Comprehensive Assessment of MS Course
Cognitive assessment in MS
Late-breaking: Role for CSF markers in autoimmune astrocytopathies
Targeted therapies for NMOSD in development
Monitoring and Treatment of Progressive MS
Challenges in diagnosing and treating progressive MS
Risk factors for conversion to secondary progressive MS
Transplantation of autologous mesenchymal stem cells
Sustained reduction in disability progression with ocrelizumab
Late-breaking: Myelin-peptide coupled red blood cells
Optimising Long-Term Benefit of MS Treatment
Induction therapy over treatment escalation
Treatment escalation over induction therapy
Influence of age on disease progression
Exposure to DMTs reduces disability progression
Predicting long-term sustained disability progression
Treatment response scoring systems to assess long term prognosis
Safety Assessment in the Post-Approval Phase
Use of clinical registries in phase 4 of DMT
Genes, environment, and safety monitoring in using registries
Risk of hypogammaglobulinemia and rituximab
Determinants of outcomes for natalizumab-associated PML
Serum immunoglobulin levels and risk of serious infections
EAN guideline on palliative care
Pregnancy in the Treatment Era
The maternal perspective: when to stop/resume treatment and risks for progression
Foetal/child perspective: risks related to drug exposure and breastfeeding
Patient awareness about family planning represents a major knowledge gap
Late-breaking: Continuation of natalizumab or interruption during pregnancy
Related Articles
June 16, 2021
Serum NfL projects development of definite MS
November 25, 2020
Therapeutic potential of anti-MOSPD2 monoclonal antibodies

© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

