The ideal induction therapy for an autoimmune disease is one “short sharp shock” of treatment with a restricted period of adverse effects, leading to long-lasting disease-specific immunological tolerance. This approach leaves the remaining immune system competent to fight infections and the patients free of infectious diseases risk.
“How close are we to this ideal model in the management of MS?” Prof. Coles asked. “There are only 3 therapies which come close to what we might consider induction therapies of MS: autologous haematopoietic stem cell therapy (aHSCT), alemtuzumab, and cladribine. I want to make a point that not all high-efficacy therapies are induction therapies by the provided definition. Clearly, natalizumab and ocrelizumab have high efficacy, but they have to be delivered continuously. It is true that induction therapies are, by definition, early therapies, given after the first identification of an abnormal immune response.”
Evidence for induction therapy
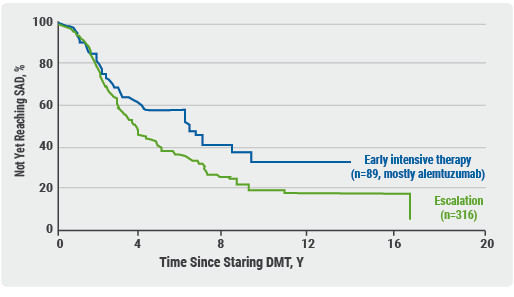
It is not possible to demonstrate immunological tolerance in MS, because the underlying pathogenic immune process is not known. “So, we have to infer the induction of tolerance by disease suppression”, Prof. Coles said. The question is whether one of the above-mentioned 3 induction therapies can lead to long-lasting disease suppression with restricted window of risk. And if so, is this better than continuous dosing with treatment escalation? Two ongoing trials are testing early intensive versus escalation in MS treatment: TREAT-MS and DELIVER-MS. “But they don’t directly address the issue of today”, Prof. Coles added, “because in the intensive therapy arm, natalizumab and ocrelizumab are included, which I don’t think count as induction therapies.” Recently published real-world data, which, according to Prof. Coles, are “exciting”, show that early, high-efficacy treatment using alemtuzumab and natalizumab slow the rate of secondary progression over 8-11 years [1]. “Nevertheless, these data cannot directly address our question”, he admitted. Another study showed that early intensive therapy, mainly using alemtuzumab, does offer benefits compared to escalation over a period of 15-16 years (see Figure 1) [2].
Figure 1. Early intensive therapy vs escalation. Median time to sustained accumulation of disability was 6.0 years for treatment with high-efficacy disease-modifying treatment (early intensive treatment) vs 3.14 years for moderate-efficacy disease-modifying treatment (escalation treatment) [2]
Prof. Coles thinks that the alemtuzumab trials are most relevant for the question at hand. In a phase 2 trial, MS patients (n=60) with a disease duration of median 1.3 years (age 32 years) either received IFN-beta or two cycles of alemtuzumab, and were subsequently followed for over 12 years. During that period, 33% of alemtuzumab-treated patients did not need a further line of therapy, 38% needed an additional three days of therapy, and 29% needed more cycles [3]. “There is prolonged suppression of relapses”, Prof. Coles concluded. “12 years after starting this induction therapy, 71% of patients either had stable or improved disability. So, we see prolonged disease suppression following alemtuzumab induction.”
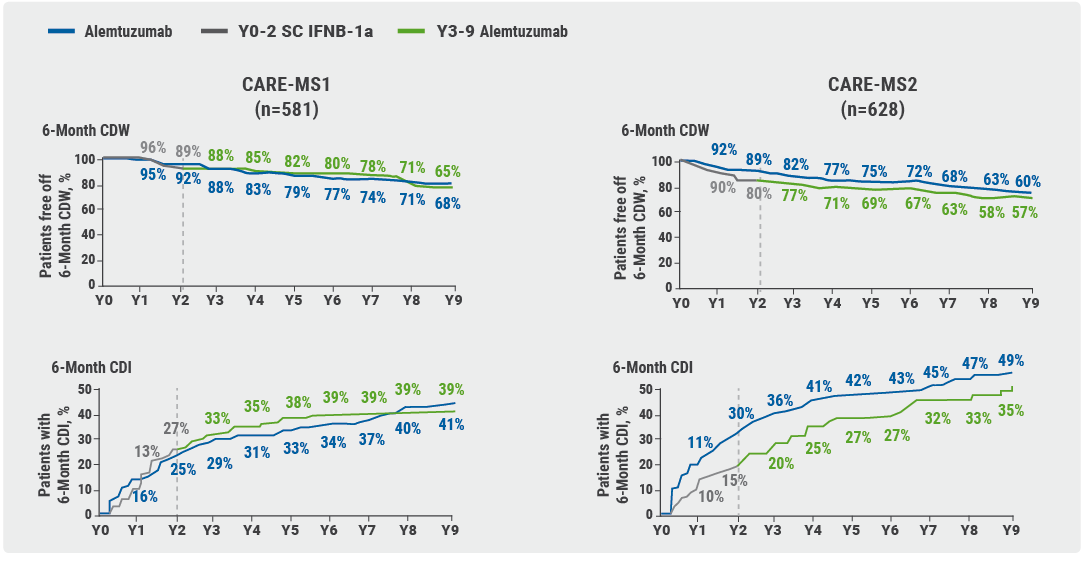
Two phase 3 trials evaluated alemtuzumab therapy in either prior untreated MS (CARE-MS1; n=581, median age 33 years, and 1.7 years since onset), or in somewhat older patients with breakthrough disease activity under disease-modifying treatment (CARE-MS2; n=628, median age 35 years, and 4 years since onset). Patients who initially received IFN-beta, were offered to switch to alemtuzumab. In CARE-MS1, 58% of patients who received alemtuzumab induction therapy needed no further treatment, 22% needed one additional cycle, and 20% multiple additional cycles. Data of 9 years of patient experience, after receiving only 8 or 11 days of alemtuzumab treatment, were presented: In previously untreated patients (CARE-MS1 trial, see Figure 2, left curves) disability progression was comparable for patients receiving alemtuzumab induction therapy versus those starting with IFN-beta and then switching to alemtuzumab [4]. “However, if you leave the patient for 2 more years, give them IFN-beta and afterwards start alemtuzumab in case of recurrent disease activity, they never recover the disability advantage (CARE-MS2 trial, see Figure 2, right curves) [5].”
Figure 2. Disability data of 9-year extension studies of CARE-MS1 and CARE-MS2 [4,5]
Safety issues
During the treatment cycles with alemtuzumab, there is serious risk of infection and infusion reactions for about a month, Prof. Coles emphasised. “After each cycle of therapy, there is a risk, with a window of 4 years, for autoimmune disease. Furthermore, patients are advised to not become pregnant for 4 months after each cycle of therapy. In an ideal situation, we have the possibility from year 5-10 to have the benefit of treatment, i.e. disease suppression, in the absence of any risks. There are also windows of disease suppression, with freedom from risks, in which it is safe to become pregnant.”
There are definitely possibilities to improve current induction therapies of MS. Firstly, one could combine induction therapy with lower-efficacy and low-risk treatments. Another option is trying to maintain immunological tolerance and reduce risk with Physician Guided Reconstitution.
- Brown JWL, et al. JAMA. 2019;321:175-187.
- Harding K, et al. JAMA Neurol. 2019;76:536-541.
- Coles A, et al. ECTRIMS 2019, abstract P651.
- Montalban X, et al. ECTRIMS 2019, abstract P974.
- Comi G, et al. ECTRIMS 2019, abstract P645.
Posted on
Previous Article
« Letter from the Editor Next Article
Tumour mutation score is a better predictor than TMB »
« Letter from the Editor Next Article
Tumour mutation score is a better predictor than TMB »
Table of Contents: ECTRIMS 2019
Featured articles
Towards a Comprehensive Assessment of MS Course
Cognitive assessment in MS
Late-breaking: Role for CSF markers in autoimmune astrocytopathies
Targeted therapies for NMOSD in development
Monitoring and Treatment of Progressive MS
Challenges in diagnosing and treating progressive MS
Risk factors for conversion to secondary progressive MS
Transplantation of autologous mesenchymal stem cells
Sustained reduction in disability progression with ocrelizumab
Late-breaking: Myelin-peptide coupled red blood cells
Optimising Long-Term Benefit of MS Treatment
Induction therapy over treatment escalation
Treatment escalation over induction therapy
Influence of age on disease progression
Exposure to DMTs reduces disability progression
Predicting long-term sustained disability progression
Treatment response scoring systems to assess long term prognosis
Safety Assessment in the Post-Approval Phase
Use of clinical registries in phase 4 of DMT
Genes, environment, and safety monitoring in using registries
Risk of hypogammaglobulinemia and rituximab
Determinants of outcomes for natalizumab-associated PML
Serum immunoglobulin levels and risk of serious infections
EAN guideline on palliative care
Pregnancy in the Treatment Era
The maternal perspective: when to stop/resume treatment and risks for progression
Foetal/child perspective: risks related to drug exposure and breastfeeding
Patient awareness about family planning represents a major knowledge gap
Late-breaking: Continuation of natalizumab or interruption during pregnancy
Related Articles
November 8, 2019
EAN guideline on palliative care
November 8, 2019
Determinants of outcomes for natalizumab-associated PML
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

