https://doi.org/10.55788/5b6039dd
In most (~85%) generalised MG patients, binding auto-antibodies to the post-synaptic acetylcholine receptor (AChR) leads to activation of the complement cascade and generation of the membrane attack complex (MAC), resulting in the destruction of the post-synaptic membrane of the neuromuscular junction [1]. Treatment with a complement inhibitor led to significant improvements of symptoms in patients with refractory generalised MG [2].
The phase 3 CHAMPION MG trial (NCT03920293) enrolled 175 patients with AChR antibody-positive (AChR Ab+) generalised MG 1:1 to receive ravulizumab infusion or placebo for 26 weeks in 85 centres worldwide [3]. The experimental group received body weight-based doses of 2,400 to 3,000 mg induction on day 1, and 3,000 to 3,600 mg maintenance dose every 8 weeks starting on day 15. All participants were allowed background treatment with an acetylcholinesterase (AChE) inhibitor and an immunosuppressant. The primary efficacy endpoint was an improvement in Myasthenia Gravis Activities of Daily Living (MG-ADL). A secondary endpoint was Quantitative Myasthenia Gravis (QMG) total score. Dr Tuan Hoang Vu (University of South Florida Physicians Group, FL, USA) presented the results.
Ravulizumab was associated with a statistically significant improvement in MG-ADL total score versus placebo (-3.1 vs -1.4; P=0.0009; see Figure). QMG total score also improved significantly compared with placebo (P<0.001), as did the proportion of patients who achieved an improvement of at least 5 points in QMG (P=0.005). Improvements in MG-ADL and QMG scores had a quick onset, within 1 week, and were maintained through week 26. No significant improvements in quality of life were detected at week 26, as measured by the Revised 15-Component Myasthenia Gravis Quality of Life score (P=0.064) or the Neuro-QOL Fatigue score (P=0.373). This result could have been influenced by the COVID-19 pandemic.
Figure: MG-ADL score improvements from week 1 through 26 [3]
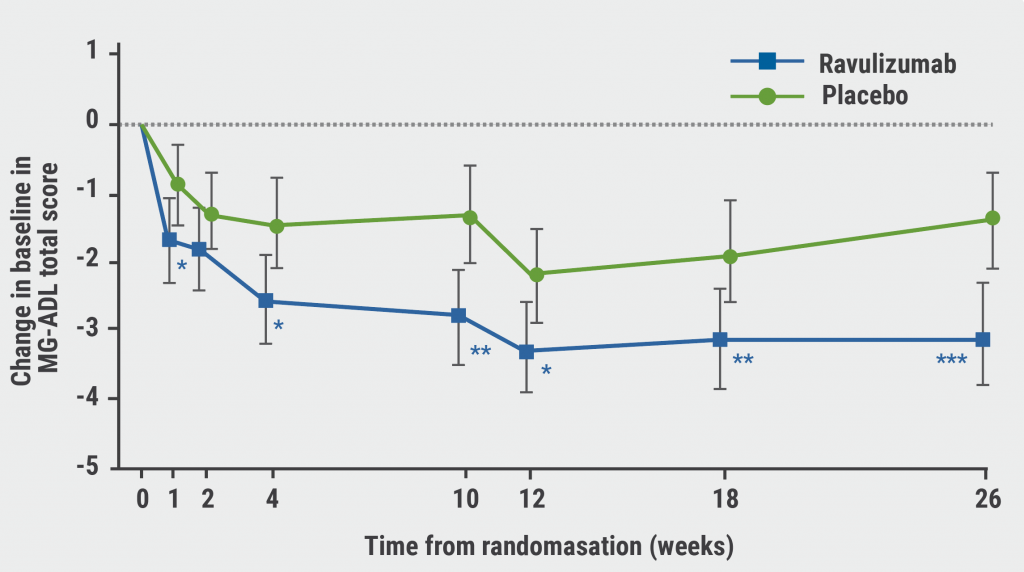
*P<0.05; **P<0.01; ***P<0.001; MG-ADL, Myasthenia Gravis Activities of Daily Living; SEM, standard error of the mean.
Adverse events (AEs) were similar in both groups. The most frequently reported AEs in the ravulizumab and placebo group were headache (18.6% and 25.8%, respectively), diarrhoea (15.1% and 12.4%), and nausea (10.5% and 10.1%). Among serious AEs, the most frequent included MG crisis (ravulizumab: 1.2%) and MG worsening (placebo: 3.4%).
After completing the randomised-controlled period, participants could enter an open-label extension (OLE), during which they received ravulizumab for an additional 26 weeks [4]. After 52 weeks, treatment effects were sustained. Patients who had originally been assigned a placebo showed immediate and sustained improvements in MG-ADL and QMG scores in the OLE, which were comparable to those in the ravulizumab group during the randomised-controlled period.
- Howard JF. Ann N Y Acad Sci. 2018;1412(1):113–128.
- Howard JF, et al. Lancet Neurol. 2017;16(12):976–86.
- Vu TH, et al. Efficacy and safety of ravulizumab in anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: phase 3 CHAMPION MG study. Clinical Trials Plenary Session, AAN 2022, 02–07 April, Seattle, USA.
- Howard JF, et al. Long-term Efficacy and Safety of Ravulizumab, a Long-acting Terminal Complement Inhibitor, in Adults with Anti-Acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis: Results from the Phase 3 CHAMPION MG Open-label Extension. S25.005, AAN 2022, 02–07 April, Seattle, USA.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Gene therapy effective in older patients with spinal muscular atrophy Next Article
Non-invasive vagus nerve stimulation for acute stroke »
« Gene therapy effective in older patients with spinal muscular atrophy Next Article
Non-invasive vagus nerve stimulation for acute stroke »
Table of Contents: AAN 2022
Featured articles
Letter from the Editor
Interview with Prof. Natalia Rost
Alzheimer’s Disease and Other Dementias
Targeting senescent cells to treat age-related diseases
Cardiorespiratory fitness protects against dementia
Safety and effects of bosutinib in Lewy body dementia
Epilepsy
“Women with epilepsy should be encouraged to breastfeed”
Fenfluramine: possible new treatment for Lennox-Gastaut syndrome
Laser interstitial thermal therapy for refractory epilepsy
Migraine
Migraine may be an important obstetric risk factor
Intranasal zavegepant safe and well tolerated in healthy adults
Telemedicine during COVID-19 pandemic highly appreciated
Multiple Sclerosis
Ublituximab versus teriflunomide in relapsing MS patients
Ketogenic diet may improve disability and quality of life
Favourable additional safety data for ofatumumab
Predicting new T2 lesions using a machine learning algorithm
Evobrutinib reduces volume of slowly expanding lesions
Sustained long-term efficacy and safety of satralizumab in NMOSD
Muscle and Neuro-Muscular Disorders
Ravulizumab in patients with generalised myasthenia gravis
Gene therapy effective in older patients with spinal muscular atrophy
Losmapimod for facioscapulohumeral muscular dystrophy
SRP-9001 for treating patients with Duchenne muscular dystrophy
Cerebrovascular Disease and Stroke
Intravenous thrombolysis after ischaemic stroke: When in doubt, leave it out?
Better outcomes with mechanical thrombectomy in elderly stroke patients
Plasma NfL levels associated with cardiovascular risk
Non-invasive vagus nerve stimulation for acute stroke
Parkinson’s Disease
Prasinezumab in Parkinson’s disease: delayed-start analysis of PASADENA trial
IPX203 versus immediate release carbidopa-levodopa
Impact of COVID-19 public health interventions
COVID-19
Cognitive, EEG, and MRI features in COVID-19 survivors
Neurological manifestations of COVID-19 worsen prognosis
New evidence for biological basis of “COVID-19 brain fog”
Related Articles
July 18, 2022
Lung transplantation after COVID-19-associated ARDS
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

