https://doi.org/10.55788/be52d394
SRP-9001 is developed for targeted expression of a shortened functional micro-dystrophin protein in skeletal and cardiac muscle. It uses the adeno-associated virus serotype rh74 (rAAVrh74) vector to deliver the micro-dystrophin-encoding gene to skeletal and cardiac muscle tissue. Efficacy and safety are evaluated in a three-part, phase 1/2 trial (NCT03769116) in patients with DMD. Participants were 4 ambulatory boys between 4 and 8 years old at study initiation with a confirmed DMD mutation between exons 18–58. They received a single intravenous infusion of SRP-9001 at an intended dose of 2.0x1014 vg/kg. The latest long-term (3-year; mean age of patients 8.2 years) safety and functional data from this study were presented by Dr Jerry Mendell (Nationwide Children's Hospital, OH, USA) [1].
North Star Ambulatory Assessment (NSAA) showed long-term overall improvements from baseline that were maintained over 3 years, indicating a durable response (see Figure). NSAA scores improved by a mean of 7.5 points overall. Dr Mendell stressed the importance of these outcomes. “There is no question about this treatment's unequivocal efficacy.” One indication was the mean change from baseline in walking 100 metres, which improved by 10.3 seconds after 3 years. NSAA improvements were generally associated with improvement in ambulation over 3 years compared with decline generally expected in untreated natural history patients.
Figure: NSAA total scores over 3 years after SRP-9001 treatment [1]
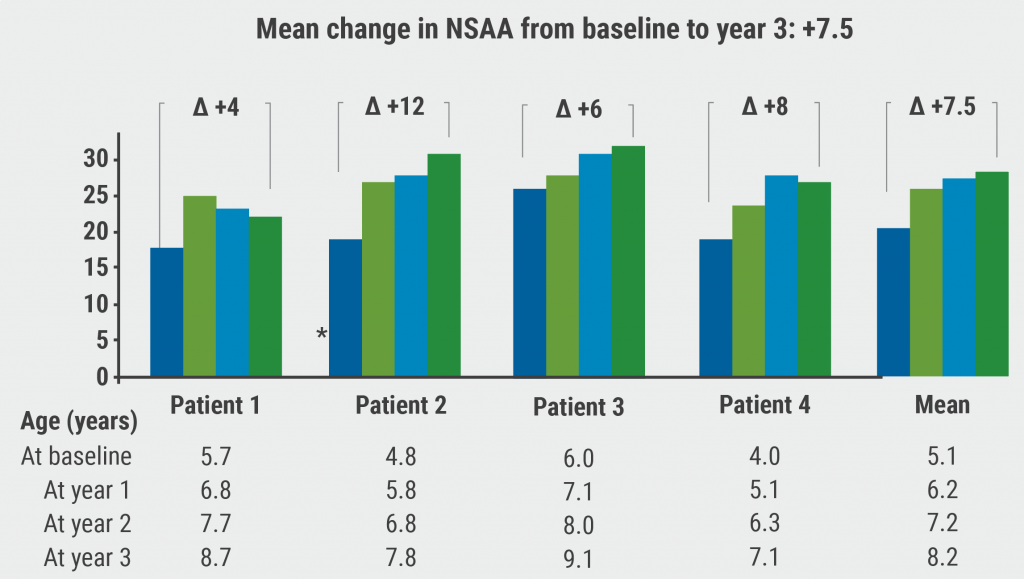
No new safety signals were detected. Safety data were consistent with the wider SRP-9001 clinical trial program. Treatment-related safety events in this study mostly occurred in the first 90 days after infusion and all resolved. These results, said Dr Mendell, reinforce the overall long-term acceptable safety profile of SRP-9001.
- Mendell JR, et al. A Phase 2 Clinical Trial Evaluating the Safety and Efficacy of SRP-9001 for Treating Patients with Duchenne Muscular Dystrophy. S23.002, AAN 2022, 02–07 April, Seattle, USA.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Ublituximab versus teriflunomide in relapsing MS patients Next Article
Losmapimod for facioscapulohumeral muscular dystrophy »
« Ublituximab versus teriflunomide in relapsing MS patients Next Article
Losmapimod for facioscapulohumeral muscular dystrophy »
Table of Contents: AAN 2022
Featured articles
Letter from the Editor
Interview with Prof. Natalia Rost
Alzheimer’s Disease and Other Dementias
Targeting senescent cells to treat age-related diseases
Cardiorespiratory fitness protects against dementia
Safety and effects of bosutinib in Lewy body dementia
Epilepsy
“Women with epilepsy should be encouraged to breastfeed”
Fenfluramine: possible new treatment for Lennox-Gastaut syndrome
Laser interstitial thermal therapy for refractory epilepsy
Migraine
Migraine may be an important obstetric risk factor
Intranasal zavegepant safe and well tolerated in healthy adults
Telemedicine during COVID-19 pandemic highly appreciated
Multiple Sclerosis
Ublituximab versus teriflunomide in relapsing MS patients
Ketogenic diet may improve disability and quality of life
Favourable additional safety data for ofatumumab
Predicting new T2 lesions using a machine learning algorithm
Evobrutinib reduces volume of slowly expanding lesions
Sustained long-term efficacy and safety of satralizumab in NMOSD
Muscle and Neuro-Muscular Disorders
Ravulizumab in patients with generalised myasthenia gravis
Gene therapy effective in older patients with spinal muscular atrophy
Losmapimod for facioscapulohumeral muscular dystrophy
SRP-9001 for treating patients with Duchenne muscular dystrophy
Cerebrovascular Disease and Stroke
Intravenous thrombolysis after ischaemic stroke: When in doubt, leave it out?
Better outcomes with mechanical thrombectomy in elderly stroke patients
Plasma NfL levels associated with cardiovascular risk
Non-invasive vagus nerve stimulation for acute stroke
Parkinson’s Disease
Prasinezumab in Parkinson’s disease: delayed-start analysis of PASADENA trial
IPX203 versus immediate release carbidopa-levodopa
Impact of COVID-19 public health interventions
COVID-19
Cognitive, EEG, and MRI features in COVID-19 survivors
Neurological manifestations of COVID-19 worsen prognosis
New evidence for biological basis of “COVID-19 brain fog”
Related Articles
September 10, 2020
Long-term safety of satralizumab consistent with double-blind periods
December 9, 2021
ECTRIMS-EAN consensus on vaccination in MS patients
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

