https://doi.org/10.55788/c6b100e2
Alopecia areata is known to exert a substantial psychosocial burden and its negative effect on the quality of life may include depression or anxiety [1,2]. Currently, an unmet need still exists for highly efficacious therapies. After favourable results in phase 2, the JAK1/2 inhibitor deuruxolitinib has now been assessed in phase 3. The results from the THRIVE-AA1 trial (NCT04518995) were presented by Dr Maryanne Senna (Lahey Hospital and Medical Center, MA, USA) [2].
The trial included 706 adult patients with a SALT score ≥50 (SALT score 0 indicates no scalp hair loss and SALD score 100 indicates complete scalp hair loss). The mean duration of the current episode at baseline was 3.7 years, the mean SALT score was 85.9, and complete or near-complete hair loss (i.e. SALT ≥95) was present in 55.8% of the participants. The participants were randomised 2:3:5 to receive a placebo, deuruxolitinib 12 mg twice daily, or deuruxolitinib 8 mg twice daily.
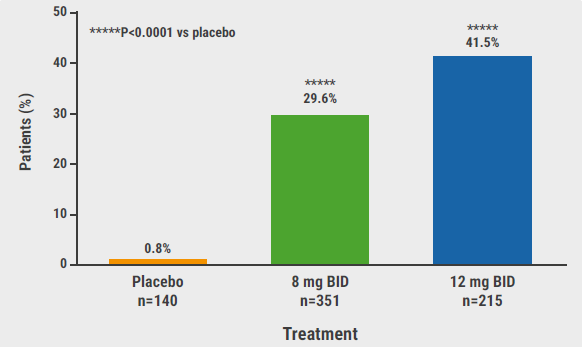
The primary endpoint of a SALT score of 20 or less at week 24 was met by both dosing regimens: 29.6% on 8 mg and 41.5% on 12 mg (P<0.0001 vs placebo for both comparisons; see Figure). Interestingly, the SALT score differences between the active agent (low and high group) versus placebo were significant as of week 8. The proportions of participants with a SALT score ≤10 at week 24, a secondary endpoint, were 0%, 20.8%, and 34.5% for placebo, deuruxolitinib 8 mg, and 12 mg, respectively. A 90% reduction in SALT at week 24 was achieved by 19.2% and 32.0% in the 2 active treatment arms (both P<0.0001 vs placebo).
Figure: Primary efficacy endpoint of proportion of patients achieving SALT score ≤20 at week 24 [1]
The safety evaluations of THRIVE-AA1 considered deuruxolitinib as mostly well tolerated since over 95% of treatment-emergent adverse events were mild-to-moderate [3]. For long-term safety, open-label extensions are underway.
All in all, the study authors rated the efficacy of deuruxolitinib in the treatment of moderate-to-severe AA as encouraging.
- Lintzeri DA, et al. Dtsch Dermatol Ges. 2022;20:59–90.
- Senna MM, et al. Efficacy of the oral JAK1/JAK2 inhibitor CTP-543 (Deuruxolitinib) in adult patients with moderate to severe alopecia areata: results from the multinational double-blind, placebo-controlled THRIVE-AA1 phase 3 trial. P41701, AAD 2023 Annual Meeting, 17–21 March, New Orleans, USA.
- King B, et al. Safety assessments in the multinational phase 3 THRIVE-AA1 trial with CTP-543 (Deuruxolitinib) in adult patients with moderate to severe alopecia areata. P42736, AAD 2023 Annual Meeting, 17–21 March, New Orleans, USA.
Posted on
Previous Article
« Biomarkers predicting response of different CSU treatments in children Next Article
Biologics in psoriasis: can they prevent joint involvement? »
« Biomarkers predicting response of different CSU treatments in children Next Article
Biologics in psoriasis: can they prevent joint involvement? »
Table of Contents: AAD 2023
Featured articles
New Developments in Dermatology
Delgocitinib shows promise as topical therapy for chronic hand eczema
Vitiligo patients maintain re-pigmentation after ruxolitinib cream withdrawal
Nemolizumab decreases lesions and itch in prurigo nodularis
Lichen planus: a future indication for baricitinib?
Atopic Dermatitis: State of the Art
As-needed ruxolitinib shows successful long-term symptom control in AD
Dupilumab: a viable option for atopic hand and foot eczema
Topical roflumilast beneficial in atopic dermatitis
IL-22 receptor blocker reduces itch and skin lesions in AD
Psoriasis: New Developments
Switching to risankizumab successful in IL-17 inhibitor non-responders
Novel, selective TYK2 inhibitor shows promise for psoriasis
Hidradenitis Suppurativa: What You Need to Know
Izokibep shows remarkably high grades of clinical response in HS
Bimekizumab could be the new up-and-comer for HS treatment
Pearls of the Posters
Biologics in psoriasis: can they prevent joint involvement?
JAK inhibitor deuruxolitinib shows encouraging hair re-growth in alopecia areata
Biomarkers predicting response of different CSU treatments in children
Related Articles
May 15, 2023
Topical roflumilast beneficial in atopic dermatitis

April 4, 2023
AAD 2023 Highlights Podcast
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

