https://doi.org/10.55788/2f9fa4ec
As IL-17A and IL-17F play an important role in various immune-mediated inflammatory diseases including HS, the principle of dual inhibition of both cytokines appeared promising to increase response rates in HS [1,2]. The pivotal, phase 3 BE HEARD I trial (NCT04242446) and BE HEARD II trial (NCT04242498) included a total of 1,014 patients with moderate-to-severe HS with at least 5 lesions and ≤20 draining tunnels [3]. Concomitant antibiotics were allowed and patients receiving antibiotics were classified as non-responders. The study treatment varied among the 4 study arms of the 2 studies, with an initial period up to week 16, and subsequent maintenance part up to week 48.
Group 1 received 320 mg of bimekizumab every 2 weeks over both study periods (Q2W/Q2W). Group 2 started with bimekizumab at 320 mg bi-weekly and switched to every 4 weeks after week 16 (Q2W/Q4W). Group 3 was kept on an every 4 weeks dose of bimekizumab 320 mg (Q4W/Q4W) from start to week 48. Group 4 began with a placebo until week 16 and continued on 320 mg of bimekizumab every second week (PCO/Q2W). The primary endpoint was the HiSCR50 response at week 16.
Baseline measures in BE HEARD I and BE HEARD II included mean age of 36.7 and 36.6 years, 63.0% and 50.7% were women, mean duration of HS of 9 and 7 years, Hurley stage 3 in 49.7% and 38.9%, and previous biologic medication in 25% and 13.2% of participants.
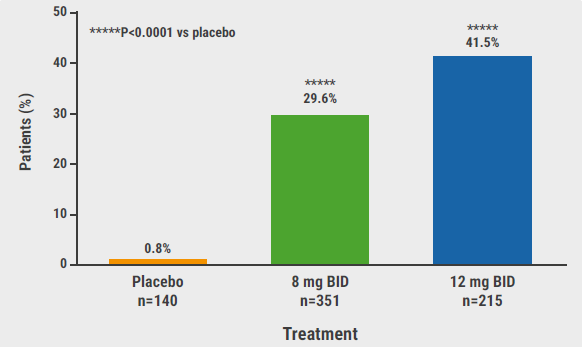
At the primary endpoint, in BE HEARD I a HiSCR50 was reached in 45.3% of participants in Q4W (P=0.03 vs placebo), 47.8% in Q2W (P=0.006 vs placebo), and 28.7% in the placebo group (see Table). The corresponding results from BE HEARD II were 53.8% (P<0.01), 52.0% (P<0.01), and 32.2%, respectively. As can be expected, the rates for achieving HiSCR75 at week 16 were overall lower. In BE HEARD II, the rates were 33.7% and 35.7% in the 2 bimekizumab arms versus 15.6% in the placebo arm (P<0.01 for both comparisons). During the maintenance period, the responses were overall sustained with a HiSCR75 in 59.8% (PCO/Q2W), 53.9% (Q4W/Q4W), 48.8% (Q2W/Q4W), and 47.3% (Q2W/Q2W) in BE HEARD 2 at week 48. The participants in the placebo group also demonstrated clinical response within the range of the other treatment arms.
Table: Results from BE HEARD I and BE HEARD II

“In particular, the HiSCR results we achieved in the BE HEARD II trial are a huge milestone for our patients,” Prof. Alexa Kimball (Harvard Medical School, MA, USA) underlined in her conclusion.
- Glatt S, et al. JAMA Dermatol. 2021;157:1279–88.
- Fletcher JM, et al. Clin & Exp Immunol 2020;201:121–34.
- Kimball A. Bimekizumab in patients with moderate-to-severe hidradenitis suppurativa: 48-week efficacy and safety from BE HEARD I & II, two phase 3, randomized, double-blind, placebo controlled, multicentre studies. S042, AAD 2023 Annual Meeting, 17–21 March, New Orleans, USA.
Posted on
Previous Article
« Switching to risankizumab successful in IL-17 inhibitor non-responders Next Article
Izokibep shows remarkably high grades of clinical response in HS »
« Switching to risankizumab successful in IL-17 inhibitor non-responders Next Article
Izokibep shows remarkably high grades of clinical response in HS »
Table of Contents: AAD 2023
Featured articles
New Developments in Dermatology
Delgocitinib shows promise as topical therapy for chronic hand eczema
Vitiligo patients maintain re-pigmentation after ruxolitinib cream withdrawal
Nemolizumab decreases lesions and itch in prurigo nodularis
Lichen planus: a future indication for baricitinib?
Atopic Dermatitis: State of the Art
As-needed ruxolitinib shows successful long-term symptom control in AD
Dupilumab: a viable option for atopic hand and foot eczema
Topical roflumilast beneficial in atopic dermatitis
IL-22 receptor blocker reduces itch and skin lesions in AD
Psoriasis: New Developments
Switching to risankizumab successful in IL-17 inhibitor non-responders
Novel, selective TYK2 inhibitor shows promise for psoriasis
Hidradenitis Suppurativa: What You Need to Know
Izokibep shows remarkably high grades of clinical response in HS
Bimekizumab could be the new up-and-comer for HS treatment
Pearls of the Posters
Biologics in psoriasis: can they prevent joint involvement?
JAK inhibitor deuruxolitinib shows encouraging hair re-growth in alopecia areata
Biomarkers predicting response of different CSU treatments in children
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com