https://doi.org/10.55788/60810b12
The quality of life of patients with AD can be significantly reduced by itch and sleep disturbances [1]. The parallel phase 3, randomised TRuE-AD1 (NCT03745638) and TRuE-AD2 (NCT03745651) trials assessed the long-term maintenance of disease and symptom control using ruxolitinib as needed in adolescent and adult patients with AD [2]. The studies included patients of ≥12 years with AD for at least 2 years who had an Investigator’s Global Assessment (IGA) score of 2 or 3 (0, clear; 1 almost clear; 2, mild; 3, moderate; 4, severe) and 3–20% affected body surface area, excluding the scalp.
After the double-blind study phase, participants who were initially randomised to ruxolitinib (either 0.75% or 1.5% cream, twice daily) subsequently remained on their regimen for the 44-week long-term safety period (i.e. as-needed treatment). “In the long-term safety period, treatment was confined to active lesions, stopped 3 days after clearance, and resumed upon recurrence,” Dr Andrew Blauvelt (Oregon Medical Research Center, OR, USA) explained. Participants were instructed to treat skin area with active AD only, but with the same regimen, twice daily. The participants did not receive concomitant or rescue treatment, and the current analysis included only those who applied ruxolitinib since day 1 (n=837).
Of the participants who applied the lower concentration ruxolitinib cream, 61.8% achieved an IGA of 0 or 1 at week 8 and 76.8% at week 52. The corresponding percentages in the participants that used the higher concentration cream were 67.1% at week 8 and 77.8% at week 52 (see Figure). Moreover, most participants (80–90%) maintained or improved their response between subsequent visits at 4-week intervals.
Figure: Change in IGA scores with as-needed treatment with 1.5% ruxolitinib cream during the long-term safety period [2]
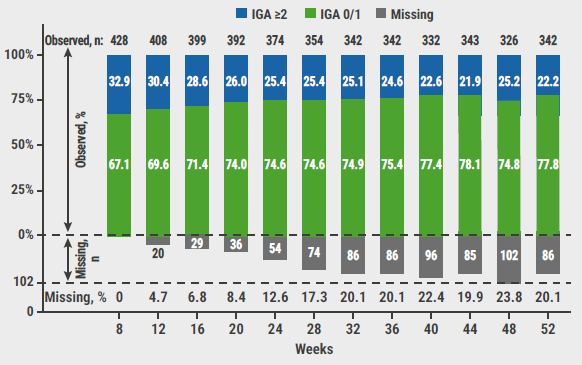
IGA, Investigator’s Global Assessment.
Most participants showed improvement in control of itch and sleep disturbance between consecutive assessments. Ruxolitinib was also well tolerated by the participants with no new safety signals.
The authors concluded that as-needed use of ruxolitinib cream is safe and effective to control AD in adults and adolescents.
- Silverberg JI, et al. J Invest Dermatol. 2015;135:56–66.
- Blauvelt A, et al. Ruxolitinib cream demonstrates maintenance of disease and symptom control with as-needed use in adults and adolescents with atopic dermatitis: pooled analysis from the long-term safety periods of two phase 3 studies. P44103, AAD 2023 Annual Meeting, 17‒21 March, New Orleans, USA.
Posted on
Previous Article
« Dupilumab: a viable option for atopic hand and foot eczema Next Article
Novel, selective TYK2 inhibitor shows promise for psoriasis »
« Dupilumab: a viable option for atopic hand and foot eczema Next Article
Novel, selective TYK2 inhibitor shows promise for psoriasis »
Table of Contents: AAD 2023
Featured articles
New Developments in Dermatology
Delgocitinib shows promise as topical therapy for chronic hand eczema
Vitiligo patients maintain re-pigmentation after ruxolitinib cream withdrawal
Nemolizumab decreases lesions and itch in prurigo nodularis
Lichen planus: a future indication for baricitinib?
Atopic Dermatitis: State of the Art
As-needed ruxolitinib shows successful long-term symptom control in AD
Dupilumab: a viable option for atopic hand and foot eczema
Topical roflumilast beneficial in atopic dermatitis
IL-22 receptor blocker reduces itch and skin lesions in AD
Psoriasis: New Developments
Switching to risankizumab successful in IL-17 inhibitor non-responders
Novel, selective TYK2 inhibitor shows promise for psoriasis
Hidradenitis Suppurativa: What You Need to Know
Izokibep shows remarkably high grades of clinical response in HS
Bimekizumab could be the new up-and-comer for HS treatment
Pearls of the Posters
Biologics in psoriasis: can they prevent joint involvement?
JAK inhibitor deuruxolitinib shows encouraging hair re-growth in alopecia areata
Biomarkers predicting response of different CSU treatments in children
Related Articles
May 15, 2023
Topical roflumilast beneficial in atopic dermatitis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

