The multicentre, open-label, phase 1/2 B-AMAZE study was presented by Dr Pratima Chowdary (Royal Free London NHS Foundation Trust, United Kingdom). Dr Chowdary explained, “The novel synthetic adeno-associated virus (AAV) capsid that we used in our study [AAVS3] has a 4 times higher liver transduction efficiency compared with a conventional one. The advantage is that with the same amount of dose or a lower dose you can potentially achieve factor IX in the normal range.”
The study aimed to restore normal coagulation, thus eliminating the need for prophylaxis and additional treatment during surgery, trauma, and activity. The investigators assessed FLT180a dose levels in an escalating/descending adaptive design to determine a dose that would achieve FIX activity levels within the normal range (50% to 150%). Dr Chowdary presented data on 10 patients with moderate-to- severe or severe haemophilia B, who were negative for neutralising AAVS3 antibodies.
Participants were treated across 4 dose levels. Two patients received 4.5x1011 vg/kg and showed FIX activity levels just below the normal range, which remained stable through 24 months. In 2 patients receiving a 7.5x1011 vg/kg dose, a modest increase was observed in FIX expression compared with a 4x1011 vg/kg dose. As part of the adaptive design, 2 additional patients received a dose of 1.5x1012 vg/kg, 1 of whom demonstrated supraphysiological FIX activity levels and was commenced on prophylactic anticoagulation after an individualised risk assessment. The anticoagulation was stopped during long-term follow-up after a multidisciplinary discussion including the patient. He then developed a thrombosis of his arteriovenous fistulae. In the final cohort, 4 patients received 9.75x1011 vg/kg, which led to FIX activity levels within the normal range through week 26 (see Table). The most common drug-related serious adverse event was transient transaminitis, which was observed in 4 patients and required supplemental immunosuppression. Immunosuppression in a refined regimen of 9.75x1011 vg/kg dose for the latest 3 patients in the highest dose group prevented transaminitis during the critical phase (4- 16 weeks). There were no bleeding episodes.
Table: B-AMAZE FIX activity levels [1]
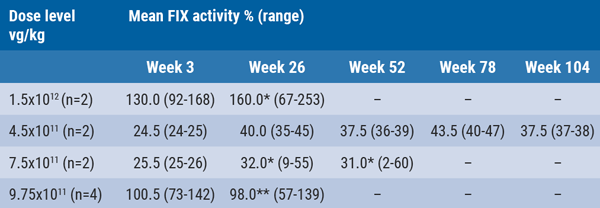
*Includes 1 patient with reduction in FIX expression following transaminitis. **Data from 2 patients and includes 1 patient with reduction in FIX expression following transaminitis.
Successful strategy to prevent transaminitis
Transaminitis is the main vector-related complication of AAV gene transfer. As Dr Chowdary explained, “transaminitis is the single most frequent risk factor for loss of effect of gene therapy. Once you have it, you lose expression of the protein and once it is lost it is practically irreversible. Therefore, we believe strongly that prophylactic immunosuppression would be the right thing for individual patients.” The B-AMAZE study was one of the first studies to develop a strategy for prevention and control of transaminitis during the high-risk period, consisting of prophylactic corticosteroid and tacrolimus therapy. No evidence of neutralising anti-FIX antibodies or infusion reactions was seen in any patients.
Dr Chowdary concluded that the FLT180a in a dose between 7.5 to 9.75x1011vg/kg achieved clinically meaningful, durable FIX activity levels in patients with haemophilia B, associated with independence from FIX replacement therapy and zero treated bleeds. However, careful dose titration seems to be necessary to identify the dose range required for sustained FIX activity in the normal range. In addition, continuous monitoring for transaminitis after the highest risk period is required for a good outcome.
Posted on
Previous Article
« Haemophilia gene therapy: progress and obstacles Next Article
Higher thrombotic risk in NSCLC patient with ALK rearrangement »
« Haemophilia gene therapy: progress and obstacles Next Article
Higher thrombotic risk in NSCLC patient with ALK rearrangement »
Table of Contents: ISTH 2020
Featured articles
Haemophilia and Rare Bleeding Disorders
Novel gene therapy leads to normal FIX activity levels in severe haemophilia B
Haemophilia gene therapy: progress and obstacles
Recombinant factor VIII safe and effective in PUPs A-LONG study
What’s New in Anticoagulation
Finding the sweet spot of anticoagulation in AF patients with ACS
Lower embryopathy risk with DOAC versus VKA during pregnancy
Higher thrombotic risk in NSCLC patient with ALK rearrangement
COVID-19 and Thrombosis
Crosstalk between inflammation and coagulation in severe COVID-19 infections
COVID-19 associated with higher VTE rates relative to influenza
Therapeutic anticoagulation not associated with lower mortality rates in COVID-19 ICU patients
COVID-19 not associated with heightened VTE risk after discharge
What’s New in Venous Thromboembolism
Residual pulmonary obstruction may predict risk of VTE recurrence
Less diagnostic delay in CTEPH diagnosis with novel algorithm
Risk of checkpoint inhibitor-associated thromboembolic events important for cancer prognosis
Pearls of the Posters
Surgical bleeding risk most important determinant of bleeding outcomes
Similar bleeding rates in patients with VTE and AF treated with DOACs
Physical rehabilitation improves health outcomes after pulmonary embolism
Guidelines adherence reduces bleeding risk after surgery and childbirth for VWD patients
Factor V Leiden mutation linked to atrial fibrillation
Increased rates of arterial thromboembolism in cancer patients
Related Articles
September 8, 2020
Haemophilia gene therapy: progress and obstacles
September 8, 2020
Higher thrombotic risk in NSCLC patient with ALK rearrangement
September 8, 2020
Recombinant factor VIII safe and effective in PUPs A-LONG study
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

