https://doi.org/10.55788/31d1f7f8
The multicentre, randomised, active-controlled AZALEA-TIMI 71 trial (NCT04755283) compared the bleeding profile of the factor XI inhibitor abelacimab with the direct oral anticoagulant (DOAC) rivaroxaban in patients with AF and a moderate-to-high risk of stroke. The participants (n=1,287) were randomised in a 1:1:1 fashion to receive 90 mg or 150 mg abelacimab, subcutaneously administered once per month, or 20 mg rivaroxaban, orally administered once daily. The primary endpoint was major or clinically relevant non-major (CRNM) bleeding. Dr Christian Ruff (Brigham and Women’s Hospital, MA, USA) presented the key findings [1].
“The trial was terminated early because the data-monitoring committee reported that abelacimab yielded a larger than anticipated reduction in the primary endpoint compared with the active comparator,” announced Dr Ruff. At the time of termination, abelacimab displayed a factor XI inhibition of >95% in both dose groups. Further, the occurrence of major or CRNM bleeding was significantly lower in the 90 mg group (HR 0.23; 95% CI 0.13–0.42; P<0.0001) and the 150 mg group (HR 0.33; 95% CI 0.19–0.55; P<0.0001) than in the rivaroxaban group (see Figure).
Figure: Primary endpoint of major or CRNM bleeding in the AZALEA-TIMI 71 trial [1]
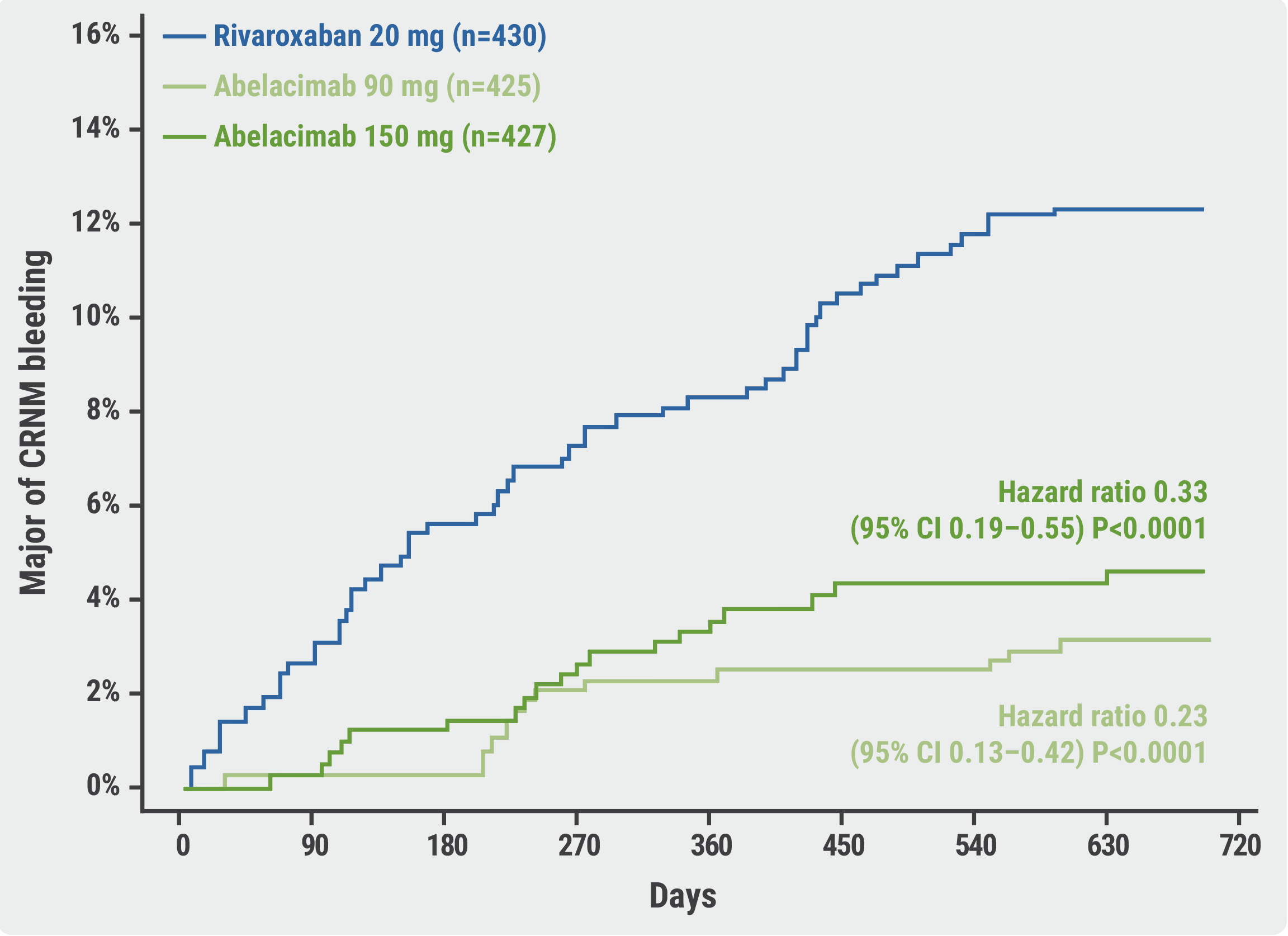 CRNM, clinically relevant non-major.
CRNM, clinically relevant non-major.
No significant difference was observed in the risk of stroke or systemic embolism between the rivaroxaban group and either the 90 mg abelacimab group (HR 1.13; 95% CI 0.41–3.12; P=0.81) or the 150 mg group (HR 1.45; 95% CI 0.55–3.80; P=0.45). “However, the rates of these events were low and were only evaluated as exploratory endpoints,” commented Dr Ruff. Finally, there were no substantial differences between the safety profiles of the 2 agents.
In summary, abelacimab 150 mg (i.e. the dose being investigated in phase 3 trials) was associated with a 67% reduction in major or CRNM bleeding compared with rivaroxaban, a decrease of 74% in major bleeding, and a reduction of 93% in major gastrointestinal bleeding. “A phase 3 trial testing abelacimab for the primary endpoint of ischaemic stroke or systemic embolism in a population of patients with AF deemed ineligible for currently available anticoagulants is underway,” Dr Ruff ended his presentation.
- Ruff CT, et al. A multicentre randomised active-controlled study to evaluate the safety and tolerability of two blinded doses of abelacimab compared with open-label rivaroxaban in patients with atrial fibrillation. LB05, AHA Scientific Sessions 2023, 11–13 November, Philadelphia, USA.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« Liraglutide may improve post-ablation outcomes in obese patients with AF Next Article
Head-to-head: Surgical embolectomy versus ultrasound-assisted thrombolysis in high-risk pulmonary embolism »
« Liraglutide may improve post-ablation outcomes in obese patients with AF Next Article
Head-to-head: Surgical embolectomy versus ultrasound-assisted thrombolysis in high-risk pulmonary embolism »
Table of Contents: AHA 2023
Featured articles
Abelacimab substantially lowers bleeding risk compared with rivaroxaban
Hot Topics in CAD/PAD
MINT: Liberal or restrictive transfusion strategy in MI with anaemia?
ORBITA-2 confirms PCI effective for symptom relief in patients with stable angina
Nicotinamide riboside shows promising trend for walking function in PAD
Pemafibrate reduces microvascular complications of PAD and T2D
Dapagliflozin improves cardiometabolic outcomes in myocardial infarction
Optimising Hypertension Outcomes
Edoxaban versus warfarin in chronic thromboembolic pulmonary hypertension
Sodium intake and blood pressure: new insights
Post-partum intervention lowers BP after hypertensive pregnancy
Biannual zilebesiran associated with substantial BP reductions
Future of Lipid-Lowering Therapies
Encouraging data for lepodisiran as Lp(a) lowering therapy
Gene editing may change the treatment landscape of hypercholesterolaemia
REPRIEVE: Mechanisms behind MACE reduction in HIV population on pitavastatin
Recaticimab may offer a solution for uncontrolled hypercholesterolaemia
Atrial Fibrillation and Sudden Cardiac Death
Abelacimab substantially lowers bleeding risk compared with rivaroxaban
Liraglutide may improve post-ablation outcomes in obese patients with AF
Single or dual cardioversion in patients with obesity and AF?
NOAH-AFNET 6: Does the duration of AHRE influence response to edoxaban?
ARTESIA: How useful is anticoagulation in subclinical AF?
Jewel IDE: High compliance rates for novel patch wearable cardioverter defibrillator
Sudden cardiac death in athletes: incidence, causes, and trends over 20 years
Miscellaneous Trials
Successful results for semaglutide in the highly anticipated SELECT trial
Can a walking intervention improve functional status and quality of life in HFrEF?
Head-to-head: Surgical embolectomy versus ultrasound-assisted thrombolysis in high-risk pulmonary embolism
Related Articles
October 30, 2023
Guidelines for Acute Coronary Syndrome
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

