Patients with metastatic HER2-positive breast cancer are typically treated with the HER2-targeted therapies trastuzumab and pertuzumab in combination with a taxane, but resistance to this regimen inevitably develops. Patients who progress on this standard therapy may then be treated with lapatinib plus capecitabine or with alternative HER2-targeted therapies, such as trastuzumab plus emtansine. Pyrotinib is an irreversible tyrosine kinase receptor inhibitor that targets HER2, as well as the related proteins HER4 and HER1. A prior phase 2 clinical trial found that pyrotinib plus capecitabine led to clinical responses in previously treated patients with HER2-positive metastatic breast cancer [1]. The phase 3 PHOEBE trial (NCT03080805) compared lapatinib plus capecitabine versus pyrotinib plus capecitabine in this patient population. Prof. Binghe Xu (Chinese Academy of Medical Sciences, China) presented the results [2].
The PHOEBE trial enrolled 267 Chinese patients with HER2-positive metastatic breast cancer who had been previously treated with trastuzumab and taxanes and up to 2 previous lines of chemotherapy in the metastatic setting. Patients were randomised 1:1 to treatment with either pyrotinib plus capecitabine or lapatinib plus capecitabine. The median follow-up was 33.2 months in the pyrotinib arm and 31.8 months in the lapatinib arm. Pyrotinib plus capecitabine significantly improved median progression-free survival compared with that for lapatinib plus capecitabine: 12.5 months versus 5.6 months (HR 0.48; P<0.0001). Median overall survival was not reached for pyrotinib and was 26.9 months for lapatinib (HR 0.69; P=0.019). Two-year overall survival rates were 66.6% and 58.8%, respectively. The benefit of pyrotinib plus capecitabine was observed in most clinically relevant predefined subgroups (including metastatic disease, trastuzumab resistance, pathological grading, visceral lesions, ECOG performance status, oestrogen and progesterone receptor status, and previous lines of chemotherapy).
“In conclusion, these updated results from PHOEBE reaffirm pyrotinib plus capecitabine as an established treatment option in this population,” said Dr Xu.
- Ma F, et al. J Clin Oncol. 2019;37:2610–2619.
- Xu B, et al. Updated overall survival (OS) results from the phase 3 PHOEBE trial of pyrotinib versus lapatinib in combination with capecitabine in patients with HER2-positive metastatic breast cancer. GS3-02, SABCS 2021 Virtual Meeting, 7–10 December.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Single-cell spatial analysis can predict response to neoadjuvant immunotherapy Next Article
Entinostat plus exemestane improves progression-free survival in Chinese patients »
« Single-cell spatial analysis can predict response to neoadjuvant immunotherapy Next Article
Entinostat plus exemestane improves progression-free survival in Chinese patients »
Table of Contents: SABCS 2021
Featured articles
Early-Stage Breast Cancer
Aromatase inhibitors outperform tamoxifen in premenopausal women
Concurrent taxane plus anthracycline most beneficial in reducing risk of breast cancer
Reduced risk of recurrence with ovarian suppression plus tamoxifen/exemestane
Metformin does not improve outcomes in patients with early-stage breast cancer
Omitting sentinel lymph node biopsy improves arm symptoms
HR-positive/HER2-negative Breast Cancer
Addition of palbociclib to standard endocrine therapy does not improve outcome in adjuvant treatment
The SERD elacestrant improves outcomes for patients unresponsive to endocrine therapy
Consistent overall survival benefit of ribociclib in advanced breast cancer
Premenopausal women benefit from adjuvant chemotherapy next to endocrine therapy
Promising anti-tumour activity of the CDK7-inhibitor samuraciclib plus fulvestrant
ctDNA is prognostic and predictive for response to ribociclib plus letrozole
Early switch to fulvestrant plus palbociclib beneficial for patients with ESR1 mutation
Triple-Negative Breast Cancer
Single-cell spatial analysis can predict response to neoadjuvant immunotherapy
Neoadjuvant pembrolizumab plus chemotherapy benefits event-free survival in TNBC
Early use of ctDNA testing can identify likelihood of relapse in TNBC
Pembrolizumab plus chemotherapy benefits patients with combined positive score ≥10
Neratinib plus trastuzumab plus fulvestrant shows encouraging clinical activity
Phase 1–3 Trials
Datopotamab deruxtecan shows promising anti-tumour activity
Trastuzumab deruxtecan outperforms trastuzumab emtansine
Nivolumab plus ipilimumab serve promising dual checkpoint inhibition
Entinostat plus exemestane improves progression-free survival in Chinese patients
Efficacy of pyrotinib plus capecitabine confirmed in previously treated patients
Basic and Translational Research
Using genomics to match treatments improves outcomes
Loss of ASXL1 tumour suppressor promotes resistance to CDK4/6 inhibitors
Inducers of ferroptosis are potential drugs to target p53-mutated TNBC cells
MAPK-pathway alterations are associated with resistance to anti-HER2 therapy
Genomic signatures of DCIS define biology and correlate with clinical outcomes
BRCA2 linked to inferior outcomes with CDK4/6 inhibitors plus endocrine therapy
Miscellaneous
Olaparib is well tolerated as an additional treatment
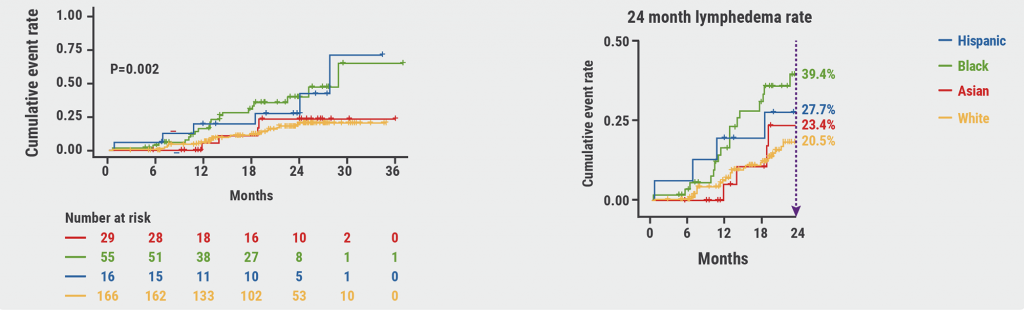
Race effects the likelihood to develop lymphoedema following breast cancer treatment
Sentinel lymph node staging is non-inferior to complete axillary lymph node dissection
One in 7 breast cancers detected during screening are overdiagnosed
Related Articles

February 17, 2022
SABCS 2021 Focus in Genomic Profiling
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

